Figure 3.
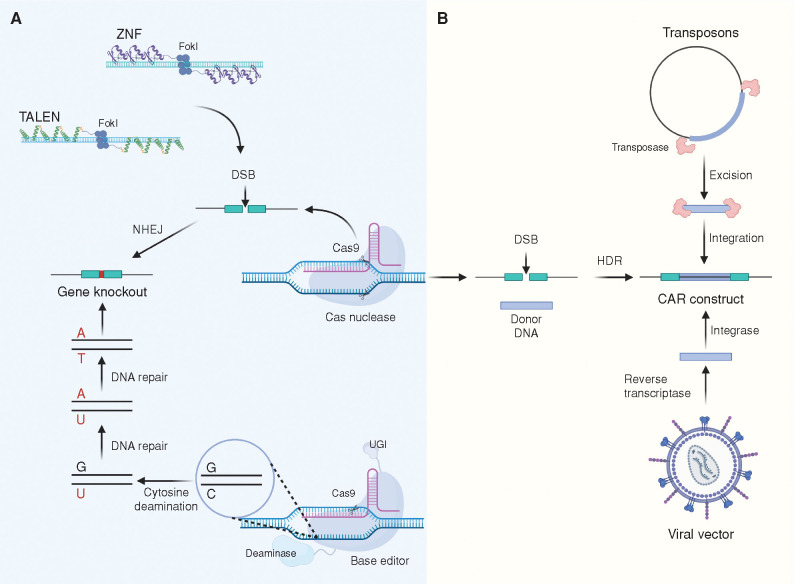
Advances in genetic modification of CAR T cells. Several gene editing tools can be used for gene delivery or gene modifications of CAR T cells. A, Knocking out genes that can interfere with the efficacy of CAR T cells is a strategy being implemented in autologous and allogeneic product manufacturing. TALENs have been used to generate universal allogeneic CAR T cells and have been tested in clinical trials (92, 148). The CRISPR-Cas9 system has also been utilized in allogeneic CAR T cell manufacturing in a similar fashion (88). Using this system, genes such as Programmed cell death protein 1 (PD-1), T-cell receptor (TCR), and beta-2 microglobulin (B2M) were knocked out, generating less alloreactive and more exhaustion-resistant T cells. Finally, the introduction of the catalytically impaired Cas9 gene editing tools enabled gene disruption without the need for double-strand breaks (DSB). The system consists of a Cas9 nickase fused to a cytidine deaminase and an uracil DNA glycosylase inhibitor (UGI). The system enables high-precision gene modifications by converting C:G to T:A base pairs. Using this technology, allogeneic CAR T cells were successfully edited to eliminate TCR, PD-1, and B2M (149). FokI, restriction endonuclease found in Flavobacterium okeanokoites; NHEJ, nonhomologous end joining. B, Alternatively, CRISPR-Cas9 can deliver the CAR gene cassette by means of homology-directed repair (HDR). The Cas9 system introduces DSBs in the presence of a donor DNA template that contains complementary homology arms capable of integrating at the site of the DSB. Using this approach, site-specific CAR integration can be achieved. This strategy enables the disruption of the TCR by targeting the T-cell receptor alpha chain constant (TRAC) locus while simultaneously integrating the CAR construct into the genome (150). These new modalities add more options to the commonly used viral vectors as well as nonviral approaches such as the piggyBac and Sleeping Beauty systems. Created with BioRender.com.