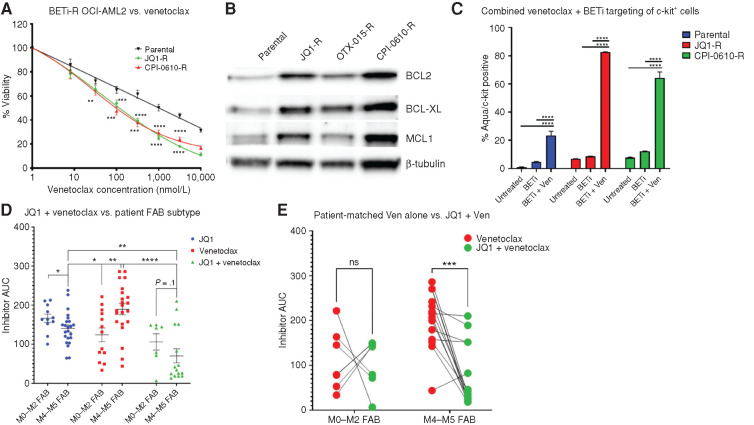
Figure 6.
BETi-R cells have increased BCL family member expression and concomitant enhanced sensitivity to BCL2i. A, BETi-naïve and BETi-R OCI-AML2 cells were subjected to titrations of the BCL2i venetoclax (Ven) for 72 hours and then assessed for viability by MTS assay. Error bars represent the standard error margin between the six replicates. P values were calculated using a two-stage linear step-up procedure of Benjamini–Krieger–Yekutieli comparing parental to BETi-R OCI-AML2 cells. B, Whole-cell extracts from naïve or BETi-R OCI-AML2 cells were subjected to immunoblot analysis using antibodies specific for BCL2L1 (BCL-XL), MCL1, and BCL2. C, Flow-cytometric analysis of treatment of BETi-naïve or BETi-R OCI-AML2 cells with vehicle, 600 nmol/L JQ1 48 hours, or 600 nmol/L JQ1 + 100 nmol/L venetoclax 48 hours versus viability by Zombie Aqua. Significance determined by two-way ANOVA with multiple comparison corrections from three replicates. OTX-015-R, OTX-015 resistant. D, Ex vivo drug sensitivities, as determined by MTS assay, in Beat AML patient samples derived from the Beat AML biorepository (3), comparing responses to JQ1, venetoclax, or JQ1 + venetoclax combined and stratified by FAB subtype (M0–M2, undifferentiated AML; M4–M5, myelomonocytic-monocytic AML). Significance determined by two-way ANOVA. N = 11 JQ1 M0 to M2, 23 M4 and M5; N = 13 venetoclax M0 to M2, 22 M4 and M5; N = 7 JQ1 + venetoclax M0 to M2, 15 M4 and M5. AUC, area under the curve. E, Sensitivity to venetoclax alone and, separately, JQ1 in combination with venetoclax, as determined by MTS assay in matched Beat AML patient samples stratified by FAB subtype. Lines connect individual patient sample responses to single-agent venetoclax (red) compared with response to JQ1 + venetoclax (green). N = 7 M0 to M2, 14 M4 and M5. Significance determined by two-way ANOVA.