Figure 1.
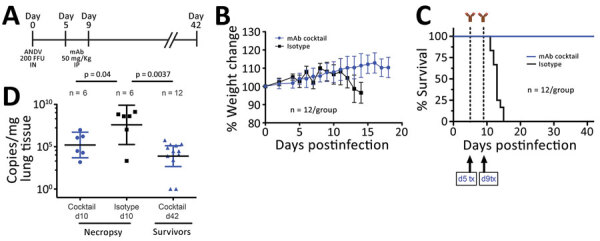
In vivo efficacy of a cocktail of human mAbs specific for ANDV glycoprotein administered at days 5 and 9 postinfection in the Syrian hamster model of hantavirus cardiopulmonary syndrome. A) Syrian hamsters were inoculated intranasally with 200 FFU of ANDV and then administered intraperitoneally a cocktail of mAb (JL16 + MIB22, 25 mg/kg each) or isotype control (50 mg/kg) on day 5 and day 9 postinfection. B) Percentage of weight change monitored until 18 days postinfection, represented as the average per group. Error bars indicate 95% CIs. C) Statistical evaluation of survival by group. Survival was evaluated at p<0.0001 by Mantel-Cox log-rank test using GraphPad Prism (GraphPad Software, Inc., https://www.graphpad.com); p<0.05 was significant. d5 tx and d9 tx indicate treatment schedule (5 and 9 days postinfection). D) ANDV RNA copies per milligram of lung tissue on day 10 postinfection (d10) and 42 days postinfection (d42). Samples were compared to a standard curve using an in vitro transcribed ANDV RNA fragment of known small segment copy number. p values by unpaired t-test using GraphPad Prism. Symbols indicate geometric means; horizontal line indicates median; error bars indicate 95% CIs. ANDV, Andes virus; FFU, focus-forming units; mAbs, monoclonal antibodies.
