Figure 3. Store-activated full-length STIM1 recruits the Orai3 M4x peptide into ER-PM junctions.
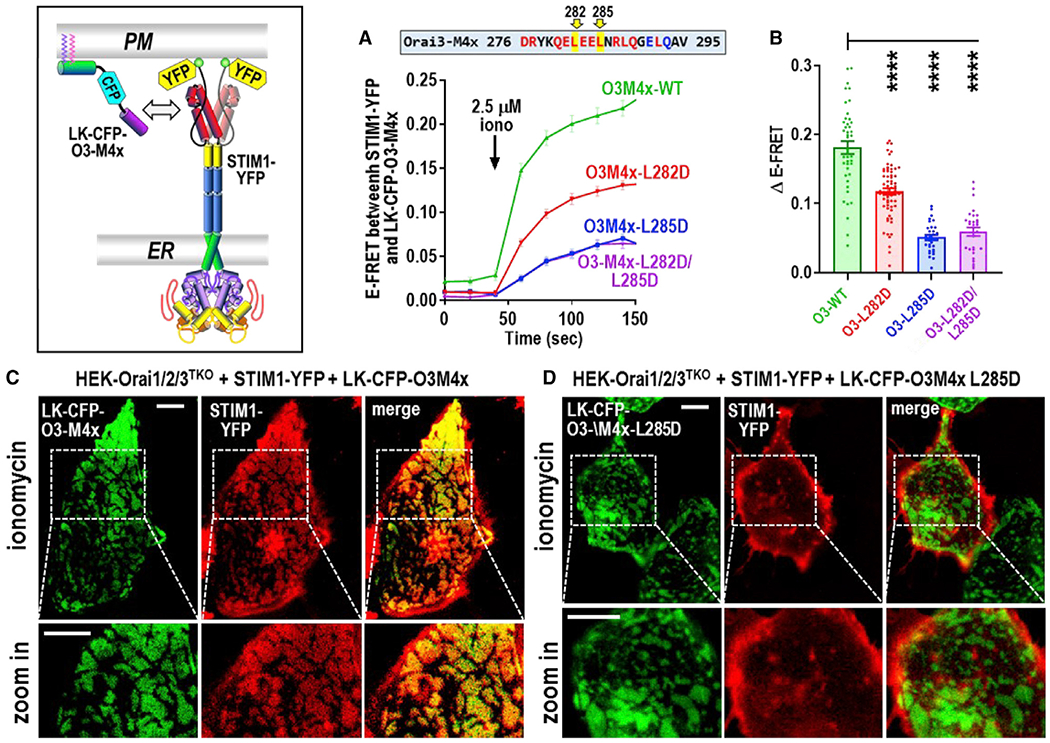
(A) Time course of E-FRET interactions between transiently expressed STIM1-YFP with LK-CFP-O3M4x constructs (either WT M4x, single L282D or L285D M4x mutants, or the L282D/L285D double mutant) in HEK-Orai1/2/3TKO cells. Store depletion was initiated by 2.5 μM ionomycin.
(B) Summary data for experiments in (A) showing the change in E-FRET from baseline (0 s) to peak after ionomycin (140 s).
(C and D) High-resolution fluorescence imaging of transiently expressed LK-CFP-O3M4x constructs with STIM1-YFP in ER-PM junctions measured at the PM layer adjacent to the coverslip in HEK-Orai1/2/3TKO cells. Images were taken 5 min after 2.5 μM ionomycin addition to deplete Ca2+ stores. In (C), LK-CFP-O3M4x WT peptide was highly localized with STIM1-YFP, and image magnification reveals a complete overlap of fluorescence in the punctal areas. In contrast, LK-CFP-O3M4x bearing the single M4x L285D point mutation (D) showed no obvious overlap with STIM1-YFP fluorescence, and magnified STIM1 punctal areas reveal no co-localized M4x construct. Images are representative of at least three independent experiments with 5-μm scale bars. One-way ANOVA was performed on E-FRET results (****p < 0.0001). Results are means ± SEM of at least three independent experiments.
