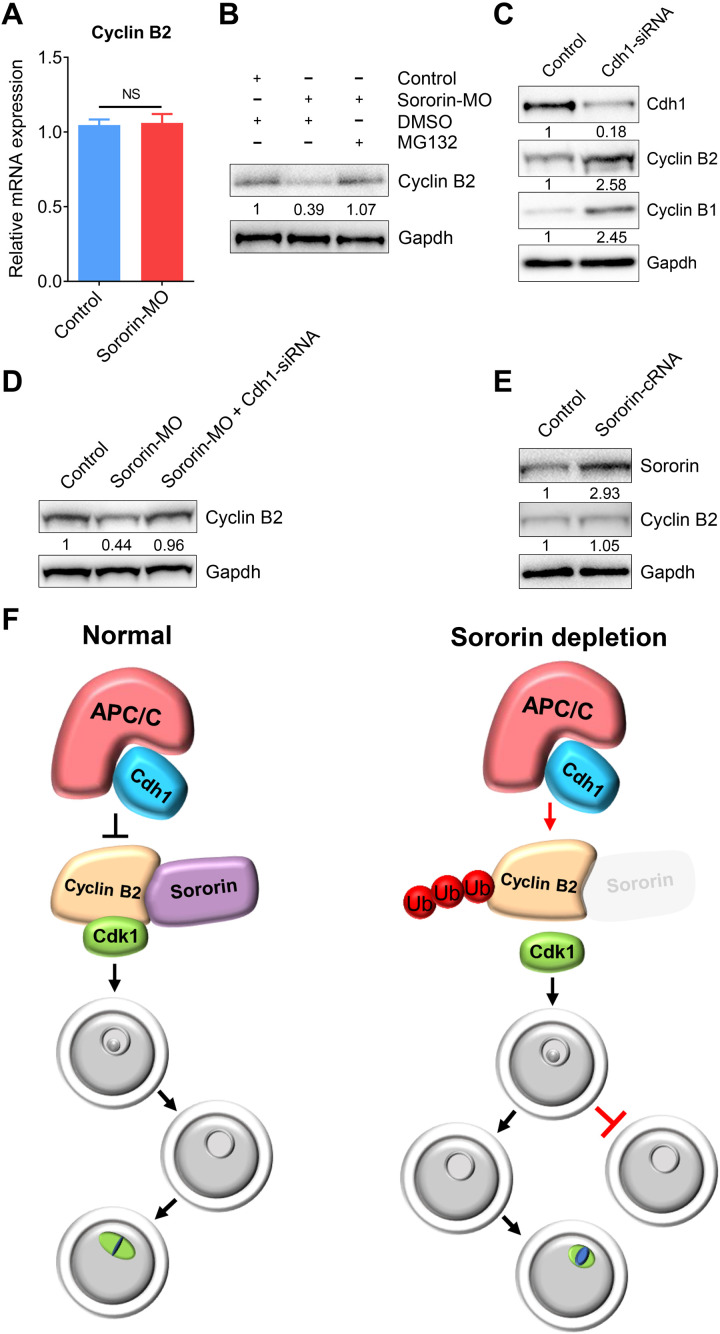
Fig. 4. Sororin protects Cyclin B2 from APCCdh1-mediated degradation.
(A) mRNA levels of Cyclin B2 were determined by reverse transcription polymerase chain reaction (RT-PCR) in control (n = 90) and Sororin-MO (n = 90) oocytes. Data were presented as mean value (mean ± SEM) of at least three independent biological replicates. NS, not significant. (B) Protein levels of Cyclin B2 in control, Sororin-MO, and Sororin-MO + MG132 oocytes. The blots were probed with Cyclin B2 and Gapdh antibodies. (C) Protein levels of Cyclin B1 and Cyclin B2 in control and Cdh1-siRNA oocytes. The blots were probed with Cdh1, Cyclin B1, Cyclin B2, and Gapdh antibodies. (D) Protein levels of Cyclin B2 in control, Sororin-MO, and Sororin-MO + Sororin-MO + Cdh1-siRNA oocytes. The blots were probed with Cyclin B2 and Gapdh antibodies. (E) Protein levels of Cyclin B2 in control and Sororin-overexpressed oocytes. The blots were probed with Sororin, Cyclin B2, and Gapdh antibodies. (F) Working model for the functions of Sororin during G2-M transition and spindle assembly in mammalian oocytes. In normal oocytes, Sororin binds to Cyclin B2 to protect it against APCCdh1-mediated degradation and thereby drives the G2-M transition and spindle assembly. However, in oocytes depleted of Sororin, either G2-M transition is inhibited or the spindle assembly during meiosis I is compromised due to the degradation of Cyclin B2.