Abstract
Background:
Screening of high-risk infants for peanut allergy (PA) before introduction is now recommended in the United States, but the optimal approach is not clear.
Objective:
We sought to compare the diagnostic test characteristics of peanut skin prick test (SPT), peanut-specific IgE (sIgE), and sIgE to peanut components in a screening population of infants before known peanut exposure.
Methods:
Infants aged 4 to 11 months with (1) no history of peanut ingestion, testing, or reaction and (2) (a) moderate-severe eczema, (b) history of food allergy, and/or (c) first-degree relative with a history of PA received peanut SPT, peanut-sIgE and component-IgE testing, and, depending on SPT wheal size, oral food challenge or observed feeding. Receiver-operator characteristic areas under the curve (AUCs) were compared, and diagnostic sensitivity and specificity were calculated.
Results:
A total of 321 subjects completed the enrollment visit (median age, 7.2 months; 58% males), and 37 (11%) were found to have PA. Overall, Ara h 2-sIgE at a cutoff point of 0.1 kUa/L discriminated between allergic and nonallergic best (AUC, 0.96; sensitivity, 94%; specificity, 98%), compared with peanut-sIgE at 0.1 kUa/L (AUC, 0.89; sensitivity, 100%; specificity, 78%) or 0.35 kUa/L (AUC, 0.91; sensitivity, 97%; specificity, 86%), or SPT at wheal size 3 mm (AUC, 0.90; sensitivity, 92%; specificity, 88%) or 8 mm (AUC, 0.87; sensitivity, 73%; specificity, 99%). Ara h 1-sIgE and Ara h 3-sIgE did not add to prediction of PA when included in a model with Ara h 2-sIgE, and Ara h 8-sIgE discriminated poorly (AUC, 0.51).
Conclusions:
Measurement of only Ara h 2-sIgE should be considered if screening of high-risk infants is performed before peanut introduction.
Keywords: Peanut allergy, screening, diagnostic test, peanut components, Ara h-2, skin prick test
Graphical Abstract
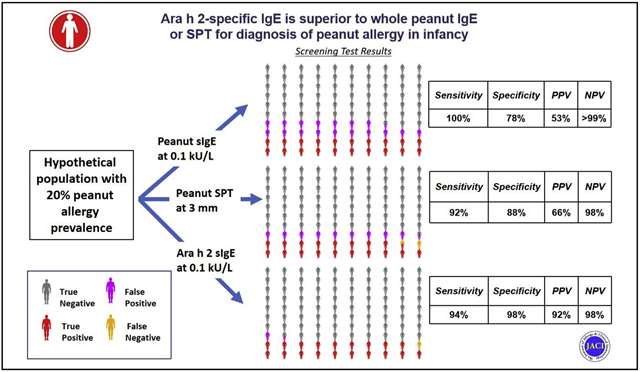
Diagnostic testing for food allergy is hampered by low diagnostic specificity of skin prick test (SPT) and specific antibody IgE (sIgE) to food extracts. The low specificity of these tests translates to low positive predictive values (PPVs) when used in populations with low prior risk of disease. For this reason, practice guidelines have generally recommended diagnostic testing only when there is strong clinical suspicion, and have advised against screening before introduction of allergenic food.1 However, new guidelines for the early introduction of peanut in the United States now recommend screening before peanut introduction in high-risk infants, with SPT as the test of choice.2 Because SPT is typically only available in allergists’ offices, they also support initial testing with sIgE, followed by SPT if the result is positive. Using peanut-sIgE avoids the need for all high-risk infants to be seen by an allergist, but it has the potential to result in a very high number of infants who end up with false-positive testing on the initial round and need further testing and a food challenge.
Component-resolved diagnostics, where sIgE to purified native or recombinant allergens is measured, may offer a more specific test.3 For peanut, component testing to the seed storage proteins Ara h 1, Ara h 2, and Ara h 3, the Bet v1 homologue Ara h 8, and, most recently, Ara h 6 is commercially available and increasingly used as an adjunct to traditional testing.4 A meta-analysis in 2016 found superiority of Ara h 2-sIgE to SPT or whole peanut-sIgE, and the authors of that meta-analysis recommended that Ara h 2-sIgE replace SPT and peanut-sIgE as the diagnostic test of choice.5 Similarly, another recent meta-analysis found superiority of Ara h 2-sIgE compared with peanut-sIgE tests.6 However, component-IgE testing is not mentioned in the recent National Institute of Allergy and Infectious Diseases (NIAID)-sponsored food allergy addendum guidelines.1,2
One reason is that it is not clear which diagnostic modality is best is that previous studies evaluating these tests have been plagued by methodological issues that tend to limit their ability to compare diagnostic tests directly.7 For example, most studies use sensitization as measured by peanut-sIgE or SPT as criteria for inclusion. By conditioning enrollment on sensitization, diagnostic specificity (the percent of test results that are negative among those without the disease) is biased downward (because most of those without allergy who do not have sensitization are excluded). Even putting aside methodological issues, there are very little data available about how these diagnostic tests perform in infancy.
The purpose of this prospective cohort study was to compare the diagnostic performance of peanut extract–based SPT, and peanut extract-sIgE, Ara h 1-sIgE, Ara h 2-sIgE, Ara h 3-sIgE, and Ara h 8-sIgE serology among high-risk infants before peanut introduction.
METHODS
Study population
Infants aged 4 to 11 months were enrolled at the Johns Hopkins Hospital and MassGeneral Hospital for Children. Inclusion criteria included no known history of peanut exposure or reaction, no history of peanut-sIgE serology or SPT testing, and at least 1 of the following potential risk factors for peanut allergy: (1) moderate-severe eczema as defined by an objective SCORing Atopic Dermatitis score of at least 25 on present or previous evaluation, or a rash that required the application of topical creams or ointments containing corticosteroids or calcineurin inhibitors and occurred on at least 7 days on 2 separate occasions, or being described by the parent or guardian as having, at any time, “a bad rash in joints or creases” or “a bad itchy, dry, oozing or crusted rash,” (2) physician diagnosis of milk, egg, or other nonpeanut allergy, and (3) a first-degree relative with peanut allergy. Details of the overall methods can be found in the companion article.8
Enrollment started at Johns Hopkins Hospital on December 21, 2016, and expanded to MassGeneral Hospital for Children on July 17, 2019. The accrual goal was 400, but because of the severe acute respiratory syndrome coronavirus 2 pandemic, enrollment was halted in March 2020 at 325 infants.
The baseline evaluation described here included SPT to peanut extract, histamine, and saline, blood draw, and, depending on the results of the SPT as outlined in the Methods section in the Online Repository at www.jacionline.org, peanut observed feeding, oral food challenge (OFC), or referral to allergy clinic with a diagnosis of peanut allergy. For logistical reasons, the observed feeding or OFC could occur up to 2 weeks after the initial screening visit, but typically occurred on the same day. Details of the methods can be found in this article’s Methods section in the Online Repository.
Statistical analysis
Study data were collected and managed using Research Electronic Data Capture (REDCap, Vanderbilt, Tenn) tools hosted at John Hopkins School of Medicine. Receiver-operator characteristic curves and area under the curve (AUC) were generated for the diagnostic tests. Diagnostic sensitivity and specificity, positive likelihood ratios, and negative likelihood ratios were calculated, with 95% CI for sensitivity and specificity estimated using the Agresti-Coull interval method.9 PPVs and negative predictive values were calculated using the estimated sensitivity and specificity under a range of expected pretest probabilities. Equality of the AUCs was tested using the method of DeLong et al.10 Nested logistic regression was used to assess whether Ara h 1-sIgE, Ara h 3-sIgE, or peanut-sIgE added to the predictive value of Ara h 2-sIgE or whether Ara h 2-sIgE added to peanut-sIgE performance. IgE values were log-transformed for these calculations. Calculated diagnostic test characteristics were applied to several hypothetical prevalence scenarios to show expected test outcomes. To generate sensitivity analyses imputing food challenge results for those who were not challenged because of SPT size, multiple imputation (10 replicates) of the food challenge result was done using age, sex, eczema status, levels of peanut-sIgE, Ara h 1-sIgE, Ara h 2-sIgE, and Ara h 3-sIgE, and SPT wheal size. All analyses were performed using STATA 14 (College Station, Tex).
The protocol was approved by the NIAID Division of Allergy, Immunology and Transplantation Clinical Research Committee, and the institutional review boards of Johns Hopkins School of Medicine and MassGeneral Hospital for Children (Partners institutional review board). The protocol was monitored by an NIAID-appointed Data and Safety Monitoring Board.
RESULTS
Participants
Population characteristics are detailed in Table I. Briefly, 321 children were enrolled (307 at Johns Hopkins and 14 at MassGeneral Hospital for Children) who had evaluable peanut allergy outcomes. The mean age at evaluation was 7.2 ± 1.7 months, 58% were males, 74% white, 8% black, 7% Asian, and 12% multiracial or of other racial background. In terms of potential risk factors, 78 had moderate-severe eczema only (as defined in the inclusion criteria), 107 had a family history of peanut allergy only, 11 had a personal history of another food allergy only, and 125 had multiple risk factors, for a total of 195 with moderate-severe eczema, 202 with family history of peanut allergy, and 59 with a personal history of food allergy other than peanut (Table I).
TABLE I.
Study population characteristics
| Characteristic | Overall (321) | Not peanut allergic (284) | Peanut allergic (37) |
|---|---|---|---|
| Age (mo), mean ± SD | 7.2 ± 1.7 | 7.2 ± 1.7 | 8.0 ± 1.7 |
| Race, % | |||
| White | 74 | 78 | 43 |
| Black | 8 | 5 | 27 |
| Asian | 7 | 5 | 16 |
| Multi/other | 12 | 11 | 14 |
| Male sex, % | 58 | 57 | 70 |
| Eczema, % | 61 | 56 | 97 |
| Family history, % | 62 | 68 | 22 |
| Physician-diagnosed food allergy, % | 18 | 17 | 30 |
| Peanut SPT wheal size (mm), median (range) | 0 (0–16.5) | 0 (0–9) | 10.3 (0–16.5) |
| Peanut s-IgE (kU/L), median (range) | <0.1 (<0.1–236) | <0.1 (<0.1–16.3) | 3.4 (0.26–236) |
| Ara h 2-sIgE (kU/L), median (range) | <0.1 (0–75.3) | <0.1 (<0.1–1) | 2.75 (<0.1–75.3) |
Observed feeding and food challenge results
Overall, 37 (11%) were found to be peanut allergic by food challenge (22), observed feeding (3), or SPT wheal size (11); the parents of 1 additional child whose peanut SPT size was 8.5 mm, peanut sIgE was 1.49 kUa/L, and Ara h 2-sIgE was 1.39 kUa/L refused the food challenge, did not introduce peanut at home, and the subject was deemed peanut allergic. Of those who were found to be not peanut allergic, 272 had a negative OFC (35) or observed feeding (237) and 12 did not consume sufficient quantity of challenge material, but introduced peanut at home without problems. One participant did not have a valid SPT result, 10 participants did not have sufficient blood for measurement of peanut-sIgE, 11 for Ara h 2-sIgE, 10 for Ara h 1-sIgE, and 13 for Ara h 3-sIgE and Ara h 8-sIgE. These participants were included in analyses as possible.
Diagnostic characteristics of peanut SPT, peanut-sIgE, and Ara h 2-sIgE for peanut allergy
As can be seen from Table II, Ara h 2-sIgE at a cutoff of 0.1 kUa/L had the best overall diagnostic test characteristics, with a receiver-operator characteristic AUC of 0.96, with a sensitivity of 94% and a specificity of 98%. Peanut-sIgE at a cutoff of 0.1 or 0.35 had somewhat better sensitivity (100% and 97%, respectively), but far worse specificity (78% and 86%), whereas SPT wheal at a cutoff of 3 mm had worse sensitivity (92%) and specificity (88%), and at a cutoff of 8 mm had substantially worse sensitivity (73%) and marginally better specificity (99%). Increasing the cutoff for Ara h 2-sIgE to 0.35 kUa/L worsened the sensitivity (89%), while improving the specificity only marginally (99%). The AUC of Ara h 2-sIgE at a cutoff of 0.1 kUa/L (0.96) was significantly higher than that of peanut-sIgE at a cutoff of 0.1 kUa/L (AUC, 0.89; P = .002), SPT wheal size at 3 mm (AUC, 0.90; P = .02), and SPT wheal size at 8 mm (AUC, 0.87; P = .02), although the comparison with peanut-sIgE at a cutoff of 0.35 was not statistically significant (AUC, 0.91; P = .08) (Fig 1).
TABLE II.
Diagnostic characteristics of peanut SPT, peanut-sIgE, and sIgE to peanut components
| Characteristic | Sensitivity (95% CI) | Specificity (95% CI) | LR+ | LR− | ROC AUC |
|---|---|---|---|---|---|
| SPT wheal size 3 mm | 92% (78%–98%) | 88% (84%–91%) | 7.68 | 0.09 | 0.90 (0.85–0.95) |
| SPT wheal size 8 mm | 73% (56%–85%) | 99% (96%–99%) | 51.81 | 0.27 | 0.87 (0.78–0.93) |
| Ara h 2-sIgE (0.1 kUa/L) | 94% (81%–99%) | 98% (95%–99%) | 43.29 | 0.06 | 0.96 (0.92–1.0) |
| Ara h 2-sIgE (0.35 kUa/L) | 89% (74%–96%) | 99% (97%–99.8%) | 81.48 | 0.11 | 0.94 (0.89–0.99) |
| Ara h 2-sIgE (1 kUa/L) | 72% (56%–84%) | 99% (97%–99.9%) | 99.31 | 0.28 | 0.86 (0.79–0.93) |
| Peanut-sIgE (0.1 kUa/L) | 100% (89%–100%) | 78% (73%–83%) | 4.60 | 0.00 | 0.89 (0.86–0.92) |
| Peanut-sIgE (0.35 kUa/L) | 97% (85%–100%) | 86% (81%–89%) | 6.7 | 0.03 | 0.91 (0.88–0.95) |
| Ara h 1-sIgE (0.1 kUa/L) | 58% (42%–73%) | 90% (86%–93%) | 5.73 | 0.46 | 0.74 (0.66–0.83) |
| Ara h 3-sIgE (0.1 kUa/L) | 33% (20%–50%) | 94% (91%–97%) | 6.04 | 0.71 | 0.64 (0.56–0.72) |
| Ara h 8-sIgE (0.1 kUa/L) | 3% (0%–0.15%) | 100% (98%–100%) | — | 0.97 | 0.51 (0.49–0.54) |
LR+, Positive likelihood ratio; LR−, negative likelihood ratio; ROC, receiver-operator characteristic.
FIG 1.
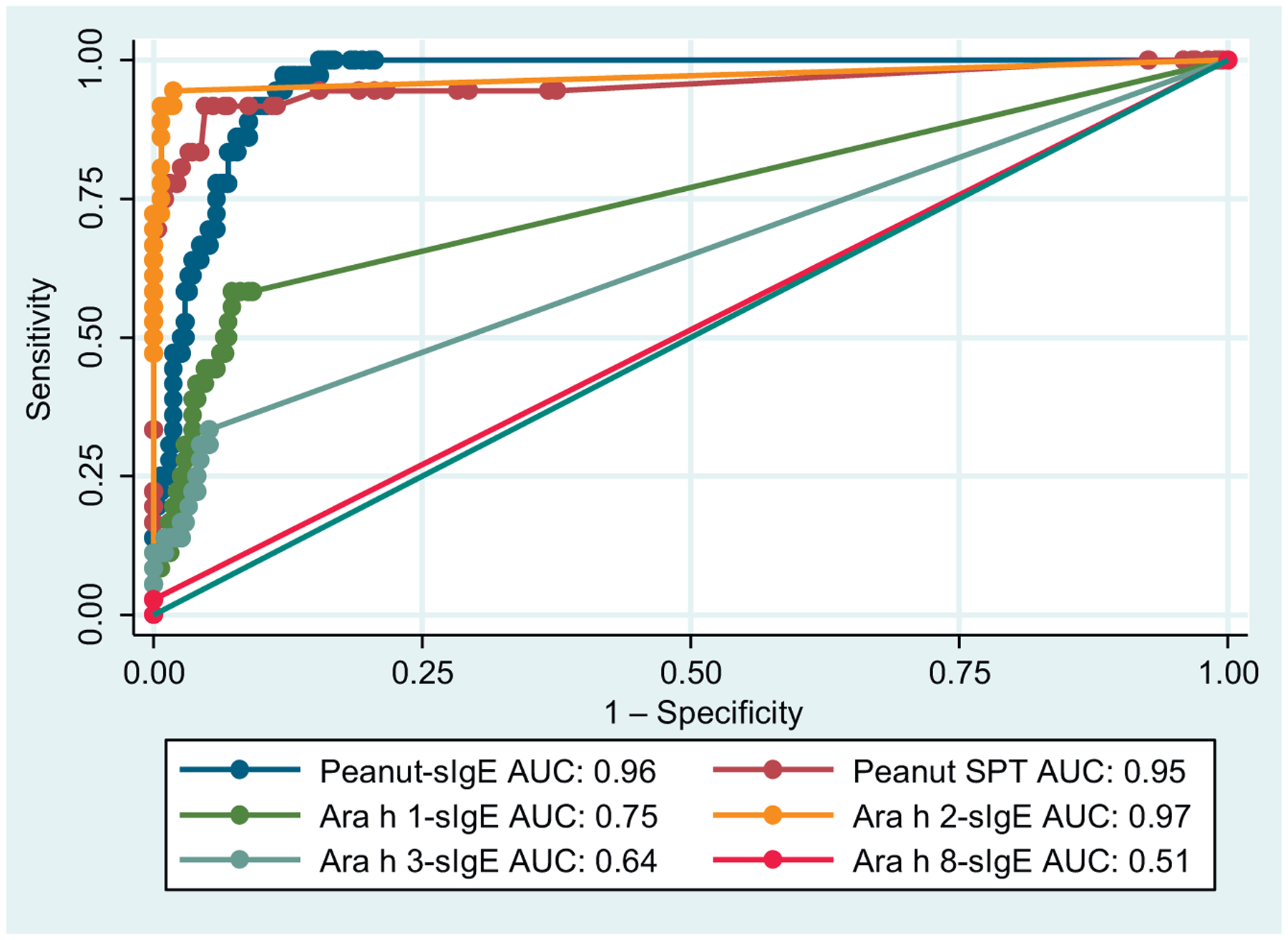
Receiver-operator characteristic curves for diagnostic tests for peanut allergy with AUC.
Utility of measurement of IgE to Ara h 1, Ara h 3, and Ara h 8
In this population, Ara h 1-sIgE or Ara h 3-sIgE had low sensitivity and relatively poor specificity for identifying peanut-allergic participants (sensitivity 58% and 33%, specificity 90% and 94%, respectively), with resulting poor AUCs (Table II). All participants with measurable Ara h 8-sIgE were peanut allergic (specificity 100%), although this identified only a very small fraction of cases (sensitivity 3%), resulting in an AUC close to a coin flip (0.51; Fig 1). There were no subjects who were peanut allergic who had a negative Ara h 2-sIgE and positive Ara h 1-sIgE, Ara h 3-sIgE, or Ara h 8-sIgE. Nested logistic regression using quantification of component-specific IgE as a continuous variable showed no added predictive value of Ara h 1-sIgE or Ara h 3-sIgE to the measurement of Ara h 2-sIgE (P = .38 and .08, respectively).
Added utility of measurement of peanut-sIgE and Ara h 2-sIgE
In a logistic regression model that included Ara h 2-sIgE, there was no improved prediction of peanut allergy status when peanut-sIgE to peanut was included (P = .33). In contrast, addition of Ara h 2-sIgE to a model with peanut-sIgE did result in significantly better predictive power (P < .001).
Approaches to screening under various disease prevalence levels
Table III presents the estimated PPV and negative predictive value of selected cutoffs for peanut-sIgE, Ara h 2-sIgE, and SPT at 2 disease prevalence levels, 2% and 20%. These prevalences were chosen because 2% approximates the rate of peanut allergy in this study among infants who had a family history of peanut allergy but did not meet the inclusion criterion of moderate-severe eczema in this study and 20% approximates the rate of peanut allergy in this study among those with moderate-severe eczema. As can be seen, when the prevalence of peanut allergy is low (2%), even a positive Ara h 2-sIgE is more likely to be a false positive (PPV, 0.49), whereas a positive peanut-sIgE will be a false positive more than 90% of the time. At higher prevalence levels of peanut allergy, such as 20%, the PPV for Ara h 2-sIgE becomes much more favorable (92%). In contrast, the negative predictive value of a negative test result is very high for peanut-sIgE and Ara h 2-sIgE at the range of prevalence between 2% and 20%, with only 2% of negative test results being false negatives at a prevalence level of 20%.
TABLE III.
PPV and NPV in selected populations based on peanut allergy prevalence
| Prevalence of peanut allergy | Test and cutoff point | PPV | NPV | Percent misclassified |
|---|---|---|---|---|
| 2% | Peanut-sIgE (0.1 kUa/L) | 0.08 | 1.00 | 22 |
| Peanut-sIgE (0.35 kUa/L) | 0.12 | 1.00 | 14 | |
| SPT wheal size 3 mm | 0.14 | 1.00 | 12 | |
| SPT wheal size 8 mm | 0.52 | 0.99 | 2 | |
| Ara h 2-sIgE (0.1 kUa/L) | 0.49 | 1.00 | 2 | |
| Ara h 2-sIgE (0.35 kUa/L) | 0.64 | 1.00 | 1 | |
| Ara h 2-sIgE (1 kUa/L) | 0.68 | 0.99 | 1 | |
| 20% | Peanut-sIgE (0.1 kUa/L) | 0.53 | 1.00 | 18 |
| Peanut-sIgE (0.35 kUa/L) | 0.63 | 0.99 | 12 | |
| SPT wheal size 3 mm | 0.66 | 0.98 | 11 | |
| SPT wheal size 8 mm | 0.93 | 0.94 | 6 | |
| Ara h 2-sIgE (0.1 kUa/L) | 0.92 | 0.98 | 3 | |
| Ara h 2-sIgE (0.35 kUa/L) | 0.96 | 0.97 | 3 | |
| Ara h 2-sIgE (1 kUa/L) | 0.96 | 0.93 | 6 |
NPV, Negative predictive value.
Several different approaches for screening and food challenge could use these diagnostic tests for screening. Table IV outlines expected outcomes of 5 different strategies. As can be seen, strategies of using an OFC either when peanut-sIgE is detectable and Ara h 2-sIgE is not or when SPT wheal size is between 3 and 8 mm result in a large portion of screened subjects requiring this procedure (18% and 13%, respectively). A strategy to challenge those with Ara h 2-sIgE between 0.1 and 1 kUa/L requires fewer procedures (5.7%), but reduces the false-positive rate only by an absolute rate of 1.3% compared with simply using Ara h 2 at 0.1 kUa/L as a cutoff point. Similarly, following an indeterminate SPT result by performing a Ara h 2-sIgE reduces the need for food challenge (8%), but it results in modestly higher rates of false positives (2%) and false negatives (1.6%) than a strategy relying on Ara h 2-sIgE alone.
TABLE IV.
Estimated diagnostic outcomes for different screening strategies, assuming a prevalence of peanut allergy of 20% in the tested population
| Diagnostic test(s) | Testing strategy | % Requiring food challenge | % False positive at end of testing | % False negative |
|---|---|---|---|---|
| Peanut SPT | Food challenge if SPT wheal size 3–8 mm; deem allergic if SPT wheal size ≥8 mm | 13 | 0.8 | 1.6 |
| Ara h 2-sIgE | If ≥0.1 kUa/L, deem allergic | NA | 1.6 | 1.2 |
| Ara h 2-sIgE | Food challenge if Ara h 2-sIgE is between 0.1 and 1 kUa/L; deem allergic if Ara h 2-sIgE >1 kUa/L | 5.7 | 0.3 | 1.2 |
| Peanut-sIgE, Ara h 2-sIgE | Food challenge if peanut-sIgE is ≥0.1 kUa/L and Ara h-2 sIgE is <0.1 kUa/L; deem allergic if Ara h-2 sIgE is ≥0.1 kUa/L | 18 | 1.6 | 0 |
| Peanut SPT followed by Ara h 2-sIgE | Food challenge if SPT wheal size 3–8 mm and Arah 2-sIgE <0.1; deem allergic if Ara h 2-sIgE ≥0.1 or SPT wheal size ≥8 mm | 8 | 2.0 | 1.6 |
Diagnostic sensitivity analyses
Multiple imputation of food challenge outcomes for participants who did not receive a food challenge was performed with 10 replicates. With the exception of SPT wheal size at a cutoff of 8 mm, where specificity changed marginally from 99% (95% CI, 96%–99%) to 98% (95% CI, 97%–100%), there were no changes to the estimated sensitivity or specificity of any of the diagnostic tests (data not shown).
Restricting the analysis to only those with moderate-severe eczema as defined by the entry criteria resulted in essentially unchanged sensitivity of the diagnostic tests, and somewhat reduced specificity (see Table E1 in this article’s Online Repository at www.jacionline.org). This was most marked for peanut SPT wheal size at a cutoff of 3 mm, where the specificity dropped from 88% to 82%, and for peanut-sIgE at cutoffs of 0.1 and 0.35 kUa/L, where the specificity dropped from 78% to 68% and 86% to 77%, respectively, whereas the specificity of Ara h 2-sIgE was largely unchanged (at 0.1 kUa/L 98% vs 97%, and at 0.35 kUa/L 99% vs 99%).
DISCUSSION
In this study of infants with potential risk factors for peanut allergy without previous peanut allergy testing, the quantification of Ara h 2-sIgE had a better overall discriminatory potential than either peanut extract–based SPT or sIgE. Although peanut-sIgE was more diagnostically sensitive, its low specificity means that over the range of prevalences of peanut allergy expected in a screening population, most patients testing positive are not actually allergic. This results in up to one-fifth of all screened patients being misclassified as peanut allergic if a cutoff point of 0.1 kUa/L is used. If screening for peanut allergy is done in infancy, a strategy involving a single assessment of Ara h 2-sIgE alone should be considered.
Many studies have shown the superior specificity of Ara h 2-sIgE compared with SPT and peanut-sIgE, including several recent systematic reviews.5,6 However, most current guidelines do not make definitive recommendations about the use of Ara h 2-sIgE or other components, and Ara h 2-sIgE is not currently recommended for screening in the NIAID-sponsored guidelines for early peanut introduction.2 One reason the use of component diagnostics has not yet been recommended is that thus far the data about comparative discriminatory ability are from studies that nearly all have major limiting biases. The most common bias that precludes clear comparisons between diagnostic tests is the use of sensitization as measured by one of the comparator diagnostic tests as an inclusion criterion for the study, a practice that falsely lowers diagnostic specificity. Here, in a study without that bias, we found higher specificity of all 3 diagnostic tests than in previous analyses. For example, in a recent meta-analysis, the diagnostic specificity of peanut-sIgE was 27% (sensitivity 93%) and that of Ara h 2-sIgE was 63% (sensitivity 92%) compared with 78% (100%) and 98% (94%), respectively, in our study.6 Even with more accurate characterization of the diagnostic ability of peanut-sIgE and SPT, we still found clinically relevant superiority of the Ara h 2-sIgE serology. Furthermore, when the population was restricted to those with moderate-severe eczema, the differences between the tests only widened, because the specificity decreased more for peanut SPT and peanut-sIgE than for Ara h 2-sIgE.
Using only Ara h 2-sIgE for screening would result in a far lower need for OFC or referral to allergy care than using peanut-sIgE, while only minimally compromising the safety of home introduction after a negative test result. Some have advocated for combining Ara h 2-sIgE and peanut-sIgE, with food challenge offered to those with some combination of positive peanut-sIgE and negative Ara h 2-sIgE.11,12 With full access to food challenge, such a strategy would ultimately minimize both false positives and false negatives, but would require food challenge for a large portion of those screened. Even screening high-risk infants (~20% likelihood of peanut allergy) with a strategy of food challenge when the peanut-sIgE is above 0.1 kUa/L and the Ara h 2-sIgE is undetectable would result in 18% of infants requiring food challenge, of which 93% would be negative, and would result in only 6% of those allergic (1.2% of the total population) avoiding home introduction in favor of food challenge. In our companion article, we found that, among infants with eczema, each added month of delayed introduction increased the odds of peanut allergy by 30%.8 Given that the marginal benefit of reacting in the office versus at home is not clear in this age group, and that the costs associated with food challenge are substantial, including monetary costs, time, burden on the health care system, and potentially delayed introduction of peanut, an approach that uses only Ara h 2-sIgE may be the most appropriate for screening.
Even though Ara h 2-sIgE has a high diagnostic specificity, the PPV of a resulting positive or negative test result is highly dependent on the pretest probability of disease. In the general population, where the prevalence of peanut allergy is approximately 2%, or among siblings of peanut-allergic children when there is not moderate-severe eczema, where we found the prevalence to be 1%,8 even Ara h 2-sIgE has a low PPV and more than half of positive test results will be false positives. Thus, our findings support the 2017 Peanut Allergy Prevention Guidelines that children with a low risk of peanut allergy, such as siblings of peanut-allergic children, should not be screened, even with Ara h 2-sIgE.2
When the pretest probability of disease is higher (ie, 20%), the PPV of Ara h 2-sIgE is relatively high (92%). However, even at this prevalence of peanut allergy, which is what we found in the current study among those deemed “high risk” by the 2017 Peanut Allergy Prevention Guidelines, there will still be almost 2% of patients overall who are falsely diagnosed with peanut allergy if Ara h2-sIgE at a cutoff point of 0.1 kU/L is used. One possible approach to further reduce the false-positive rate would be to perform an OFC when the Ara h 2-sIgE is positive but low. If an OFC were performed when Ara h 2-sIgE is between 0.1 and 1 kUa/L at a population prevalence of 20%, this would reduce the overall percentage of patients with false positives from 1.6% to 0.3%, but it would require that more than 5% of all screened patients be challenged, of whom more than 75% would be allergic. The decision about whether to perform challenges at low Ara h 2-sIgE levels should incorporate pretest probability of disease, family preference, and other factors such as availability of a food challenge.
Typically, component sIgE is ordered as a panel.13 Whether the other peanut components have any value in screening or diagnosing peanut allergy in this age group has not previously been clear. Indeed, it is not even clear whether providers should use sensitization to these components as indicators of allergy, or conversely, as indicators of clinically irrelevant sensitization. Here, we found that there was no added utility to measuring Ara h 1-sIgE, Ara h 3-sIgE, or Ara h 8-sIgE above the measurement of Ara h 2-sIgE. Despite evidence in older children and adults that sensitization to Ara h 8, a Bet v 1 homologue, might identify peanut-sensitized subjects who have birch sensitivity and are not truly allergic to peanut, we found no infant sensitized to Ara h 8 who was not peanut allergic. From other work, it appears that sensitization patterns among peanut-allergic patients are dependent on age and geography, so it is possible that in other populations, measuring IgE to these components could aid in the diagnosis of peanut allergy.14,15 In our study, in a screening population of infants in the United States, it appears that measuring these components adds to cost and provides no useful additional information.
For a patient who is already being seen by an allergist, SPT offers a faster turnaround time and can be less expensive than sIgE testing. The NIAID-sponsored guidelines on early introduction currently recommend food challenge when the SPT wheal size is between 3 and 8 mm, with home introduction below 3 mm and peanut allergy diagnosis when SPT wheal size is 8 mm or higher. After food challenge, this strategy results in relatively few false-positive or false-negative diagnoses if the pretest probability is sufficiently high, but we estimate that it would require 13% of screened infants to be challenged. If indeterminate SPT results (wheal size 3–8 mm) were followed by Ara h 2-sIgE, we estimate that the number of OFCs required would decrease to 8% of screened infants, but both the false-positive and false-negative rates of the testing scheme would be somewhat higher than if Ara h 2-sIgE were used alone with no food challenge. Whether it is appropriate to include SPT in a screening strategy depends on the ready availability of the food challenge and the relative difficulty of obtaining serology versus performing an SPT.
The most important limitation of this analysis is that there was a subset of infants who did not receive a food challenge because of a large SPT wheal size. This has the potential to inflate the specificity of all tests, but most substantially the SPT itself. However, we did challenge 9 consecutive infants in this range, all of whom failed the challenge, and our sensitivity analyses using multiple imputation to model the results of the 11 subjects deemed allergic by SPT did not change our estimates of sensitivity or specificity for any diagnostic test cutoff point, except for SPT wheal size at 8 mm, where the specificity dropped from 99% to 98%. Importantly, because all the infants who were not challenged had higher levels of peanut-sIgE and Ara h 2-sIgE, this should not change the comparative analysis of the diagnostic tests. Another limitation is that we did not measure sIgE to other peanut components such as Ara h 6 or Ara h 9. In addition, SPT is user dependent, and may perform better or worse in other hands. These limitations are balanced by the strength of the study involving the lack of selection bias with respect to sensitization status, which allows us to directly compare the diagnostic tests.
Conclusions
If screening of any subsets of infants before peanut introduction is going to be performed, using an Ara h 2-sIgE serology as the sole screening test should be considered. SPT with challenge at intermediate levels is a more labor-intensive strategy and is likely to result in slightly more false negatives, but it is an alternate approach for a patient already in the allergists’ office or to avoid blood draw. The US Peanut Allergy Guidelines included peanut-sIgE as an option to ensure that high-risk infants could safely introduce peanut while limiting the need for specialist care before introduction. Replacing peanut-sIgE with Ara h 2-sIgE in screening guidelines would substantially reduce the number of children who are false positive to peanut and require referral to allergy care and food challenge, while allowing for nonspecialists to provide the screening.
Supplementary Material
Clinical implications:
In infants, Ara h 2-sIgE was superior to peanut SPT or sIgE for diagnosis of peanut allergy. Screening using only Ara h 2-sIgE should be considered.
Acknowledgments
We acknowledge Mharlove Andre for assistance with recruitment and data collection.
This study was funded by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (grant no. 1U01AI125290). This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by the National Center for Advancing Translational Sciences (NCATS) (grant no. UL1 TR003098), a component of the NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH. The project described was supported by grant number 1UL1TR002541-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the NCATS, or the NIH. J.D. is funded by the Pearl M. Stetler Fund.
Disclosure of potential conflict of interest:
C. Keet receives royalties from Up to Date. W. Shreffler has served on the Scientific Advisory Board of Aimmune Therapeutics, and as an advisor to Food Allergy Research and Education (FARE), Buhlmann Laboratories AG, and Sanofi Pasteur. R. Wood receives research support from FARE, Aimmune, DBV, Astellas, Regeneron, Sanofi, and HAL-Allergy, and royalties from Up to Date. M. Pistiner has served as a consultant for AAFA, kaléo, and DBV Technologies; received funding from kaléo, DBV Technologies, and National Peanut Board; and is cofounder of AllergyHome and Allergy Certified Training. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- AUC
Area under the curve
- NIAID
National Institute of Allergy and Infectious Diseases
- OFC
Oral food challenge
- PPV
Positive predictive value
- sIgE
Specific IgE
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: Disclaimer: Dr Togias’ authorship of this report does not constitute endorsement by the US National Institute of Allergy and Infectious Diseases or any other US government agency.
REFERENCES
- 1.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126:S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR Jr, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 2017;139:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes de Oliveira LC, Aderhold M, Brill M, Schulz G, Rolinck-Werninghaus C, Clare Mills EN, et al. The value of specific IgE to peanut and its component Ara h 2 in the diagnosis of peanut allergy. J Allergy Clin Immunol Pract 2013; 1:394–8. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018;141:41–58. [DOI] [PubMed] [Google Scholar]
- 5.Klemans RJ, van Os-Medendorp H, Blankestijn M, Bruijnzeel-Koomen CA, Knol EF, Knulst AC. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy 2015;45: 720–30. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson C, Berthold M, Mascialino B, Orme ME, Sjolander S, Hamilton RG. Accuracy of component-resolved diagnostics in peanut allergy: systematic literature review and meta-analysis. Pediatr Allergy Immunol 2020;31:303–14. [DOI] [PubMed] [Google Scholar]
- 7.Keet CA. A call to improve standards for reporting of diagnostic test research in allergy. J Allergy Clin Immunol 2016;137:1761–3. [DOI] [PubMed] [Google Scholar]
- 8.Keet C, Pistiner M, Plesa M, Szelag D, Shreffler W, Wood R, et al. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. J Allergy Clin Immunol 2021[in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai WY, Chi Y, Chen CM. Interval estimation of binomial proportion in clinical trials with a two-stage design. Stat Med 2008;27:15–35. [DOI] [PubMed] [Google Scholar]
- 10.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 11.Caubet JC, Sampson HA. Beyond skin testing: state of the art and new horizons in food allergy diagnostic testing. Immunol Allergy Clin North Am 2012;32:97–109. [DOI] [PubMed] [Google Scholar]
- 12.Martinet J, Couderc L, Renosi F, Bobee V, Marguet C, Boyer O. Diagnostic value of antigen-specific immunoglobulin E immunoassays against Ara h 2 and Ara h 8 peanut components in child food allergy. Int Arch Allergy Immunol 2016;169:216–22. [DOI] [PubMed] [Google Scholar]
- 13.Valcour A, Jones JE, Lidholm J, Borres MP, Hamilton RG. Sensitization profiles to peanut allergens across the United States. Ann Allergy Asthma Immunol 2017;119:262–6.e1. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaou N, Custovic A. Molecular diagnosis of peanut and legume allergy. Curr Opin Allergy Clin Immunol 2011;11:222–8. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Blanca A, Aranda A, Blanca-Lopez N, Perez D, Gomez F, Mayorga C, et al. Influence of age on IgE response in peanut-allergic children and adolescents from the Mediterranean area. Pediatr Allergy Immunol 2015;26:497–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


