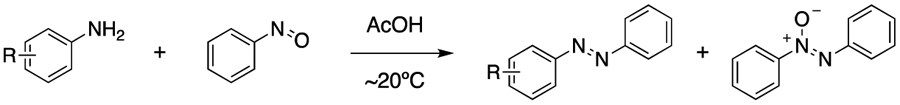
Table 1.
Reaction of substituted anilines with nitrosobenzene. Reagents and conditions: appropriate aniline (1.0 eq.), nitrosobenzene (1.0 eq.), AcOH (0.5 M), ambient temperature, 24 h.
| Entry | R | Calculated Eoxa | σp+ | Azobenzene % Yieldb | Azoxybenzene % Yieldb |
|---|---|---|---|---|---|
| 1 | p-NMe2 | 0.18 | −1.70 | 34 | 45 |
| 2 | p-NH2 | 0.38 | −1.30 | 28 | 43 |
| 3 | o-NH2 | 0.73 | N/A | 8 | 91 |
| 4 | p-OMe | 0.77 | −0.78 | ≥95 | 5 |
| 5 | 2,6-dimethoxy | 0.77 | N/A | 46 | 35 |
| 6 | p-NHCO2tBu | 0.80 | N/A | 87 | 6 |
| 7 | p-OH | 0.83 | −0.92 | 34 | 69 |
| 8 | o-OMe | 0.93 | N/A | 82 | 13 |
| 9 | p-Me | 0.97 | −0.31 | 95 | ≤5 |
| 10 | o-NHCO2tBu | 1.04 | N/A | 85 | 12 |
| 11 | o-Et | 1.08 | N/A | 77 | 20 |
| 12 | p-I | 1.16 | 0.14 | ≥95 | ≤5 |
| 13 | H | 1.20 | 0.00 | ≥95 | ≤5 |
| 14 | o-Br | 1.46 | N/A | 49 | 8 |
| 15 | p-CO2Me | 1.49 | 0.49 | 82 | 6 |
| 16 | 2,6-difluoro | 1.55 | N/A | 12 | ≤5 |
| 17 | p-CN | 1.63 | 0.66 | 64 | 7 |
| 18 | p-CF3 | 1.67 | 0.61 | ≥95 | ≤5 |
| 19 | o-NO2 | 1.88 | N/A | ≤5 | ≤5 |
| 20 | p-NO2 | 1.90 | 0.79 | 19 | ≤5 |
Volts vs. SHE.
Yield determined by 1H NMR using dibromomethane as an internal standard.
N/A = not available.