Abstract
Background:
Clinical outcomes suggest that post-oncologic reconstruction with fat grafting yields cumulative incidence curves of recurrence comparable to other breast reconstruction procedures, however results from experimental research studies are discordant. In this study, a novel animal model of residual cancer cells in mouse mammary pads was developed to test whether lipofilling impacts the probability of post-mastectomy locoregional recurrence of breast cancer after breast conserving surgery.
Methods:
Mammary fat pads of female NOD-SCID gamma mice were each injected with MCF7 cells in matrigel. Tumors were allowed to engraft for 2 weeks, after which time either sterile saline (N=20) or human fat graft (N=20) was injected adjacent to tumor sites. After 8 weeks, tumors were assessed for volume measurement, histological grade, Ki67 positivity and metastatic spread.
Results:
Animals receiving lipofilling after tumor cell engraftment had lower tumor volume and mass (p=0.046 and p=0.038, respectively). Macroscopic invasion was higher in saline group. Histological grade was not significantly different in the two groups (p=0.17). Ki67 proliferation index was lower in tumors surrounded by fat graft (p=0.01). No metastatic lesion was identified in any animal.
Conclusion:
Adipose transfer for breast reconstruction performed in the setting of residual breast tumor in a clinically relevant animal model did not increase tumor size, proliferation, histological grade or metastatic spread. This study supports of oncologic safety of lipofilling as part of the surgical platform for breast reconstruction after cancer therapy.
Keywords: Breast Reconstruction, Adipose Transfer, Breast Cancer, Mammaplasty
BACKGROUND
Early detection and improved treatments have increased breast cancer survival rate by 38% since 1989, however, this has led to an increasing proportion of American women who require some form of breast reconstruction1. Reconstruction with autologous tissues, implants, or a combination of both, has a number of benefits particularly related to increase in quality of life. As such, breast reconstruction following mastectomy or breast conserving surgery is a key component of the multidisciplinary cancer treatment with insurance coverage for such procedures mandated by the Women’s Health Act of 19982–4. Breast reconstruction therapies typically consist of tissue flap procedures or silicone implants, however recent reports indicate that 62% of American and 69% of British certified Plastic Surgeons perform fat graft alone or as an adjunctive therapy to improve outcomes in patients who have undergone flap or implant procedures5–6. Analyses of clinical outcomes suggest that post-oncologic reconstruction with lipofilling yields cumulative incidence curves of local recurrence comparable to other breast reconstruction procedures with a possible exception of intraepithelial neoplasia, which is not confirmed in a long-term basis7–12. However, concerns regarding the oncologic safety of adipose transfer have been raised and published guidelines recommend postponing indications of adipose transfer for breast reconstruction to a moment when patients are considered cured and advocate for careful patient selection and close follow-up13–16.
Adipose tissue has a multitude of functions including a strong secretory capacity with paracrine and endocrine effects and is a source of multipotent stem cells and immune cells, which may promote angiogenesis and tumorigenesis during the wound healing response17–19. Experimental studies combining cultured adipose-derived stem cells (ASCs) and breast cancer cells in co-culture show increased tumor cell proliferation, invasive potential, metastatic spread, epithelial to mesenchymal transition and angiogenesis20–21. Published clinical papers however are contradictory to this in vitro results and, thus, there is a clear dissociation between in vitro and clinical research in terms of fat graft oncological safety.
To critically study the impact of autologous fat grafting on local breast cancer recurrence, our group has developed humanized mouse models using breast cancer cell lines engrafted in the mouse mammary fat pad. Data presented in our recent publication using the triple negative MDA-MB-231 and HER2 positive, luminal B-like BT 474 cell lines, are in line with clinical studies and our preliminary results suggest that fat tissue does not increase breast cancer cell proliferation in vivo.22 The aim of this study is to expand on our previous work with the mouse breast cancer model to include a third type of breast cancer cell, the MCF7 luminal A-like cell line. Further, we looked at the impact of grafted lipoaspirate on a range of tumor cell doses to determine the impact of adipose tissue on tumor presentation and histologic analysis.
METHODS
Animals
Forty female 8–10 weeks NOD-SCID gamma mice (Jackson Laboratory - Bar Harbor, ME, USA - stock number 005557), 22.1+/−1.8g, were used in this study. Animals were housed 5 per cage in a pathogen-free environment, 12/12 hour light-dark cycle and had water and food ad libitum. All procedures and animal care were performed in accordance with protocol approved by University of Pittsburgh Institutional Animal Care and Committee (protocol #1506677). NOD-SCID gamma mice are considered the best animal model for xenografts and were chosen for this study because one of the key features of the present model was to include only human cells both in breast cancer and fat graft.
Xenograft of breast cancer cell line MCF-7
Animals were injected with MCF-7 breast cancer cells, purchased from American Type Culture Collection (Manassas,VA, USA). Prior to use, cells were culture expanded in DMEM, 10% fetal bovine serum, 1% Penicilin/Streptomicin (Penicilin 10000U/ml and Streptomicin 10000μg/ml) and 0.5% anfotericin (amphotericin-B 250μg/ml). All cell culture products were purchased from BioWhittakerTM, Lonza™, Basel, Switzerland, unless otherwise stated.
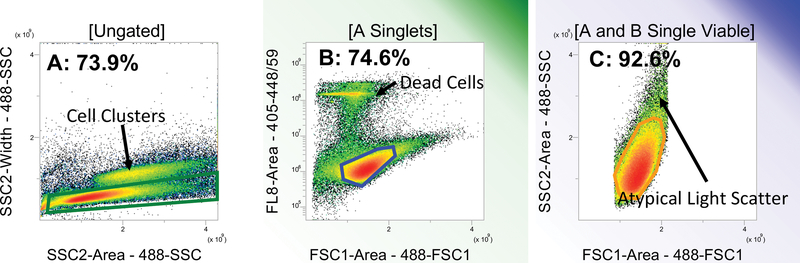
On the day of injection, cells which were 70% confluent were trypsinized and resuspended in fresh medium. Two cellular aliquots were prepared: tumorigenic cells and accessory cells, which were non-proliferative cells included to minimize loss of tumorigenic cells during handling and delivery. Accessory MCF-7 cells were prepared by irradiating cells at 10,000rads by a Gammacell 1000™ irradiator (Nordion™, Otawa, Canada); then, counting and distributing at 10,000 cell per 25μL media into each well of a 96-well, V-bottom, polypropylene plates. Non-irradiated tumorigenic cells were then counted by Moflo Astrios (Beckman Coulter™ Indianapolis, IN, USA) and 1,000 cells were added to each well of the same plate. Media volume was considered negligible. Cell sorting was done by Blue 488nm laser to obtain forward scatter-FSC and side scatter-SSC; and a violet 405nm laser was used to excite DAPI for eliminating dead or apoptotic cells. Sort logic and gates are demonstrated in Figure 1. Cells were sorted through a 100μm tip under a pressure of 29psi. Cytometer was set up to detect 1,000 events per second. After cell sorting, 25μl of Matrigel™ (Corning™, Tewksbury MA) was added to each well (total volume=50μl) and plates were kept on ice until time of injection.
Figure 1:
Sort logic for live MCF7 cells sorting into plates. Left to right: single cells (73.9% of total events) are discriminated from cell clusters (A); DAPI exclusion is used as criterion for viable cells (74.6% - B); final sort gate includes only viable single cells with typical light scatter (92.6% - C). Percentages represent percent of the gated population.
Animals were anesthetized with isoflurane 3% and 70% ethanol was used for anti-sepsis. Cells and matrigel (50μL) were aspirated into a 0.5ml syringe connected to a 28G needle (1/2 mL BD Lo-Dose™ U-100 insulin syringe with 28 G × 1/2 in, Becton Dickinson™, Franklin Lakes, NJ, USA) and injected into 4 unique sites in mammary fat pads including the 2nd right, 2nd left, 4th right and 4th left. After xenograft injections, animals received a subcutaneous nuchal dose of ketoprofen 5mg/kg and were closely observed for 72 hours for signs of pain or distress. Animals were assessed daily to confirm general health condition and palpated once a week to assess tumor growth.
Fat graft intervention
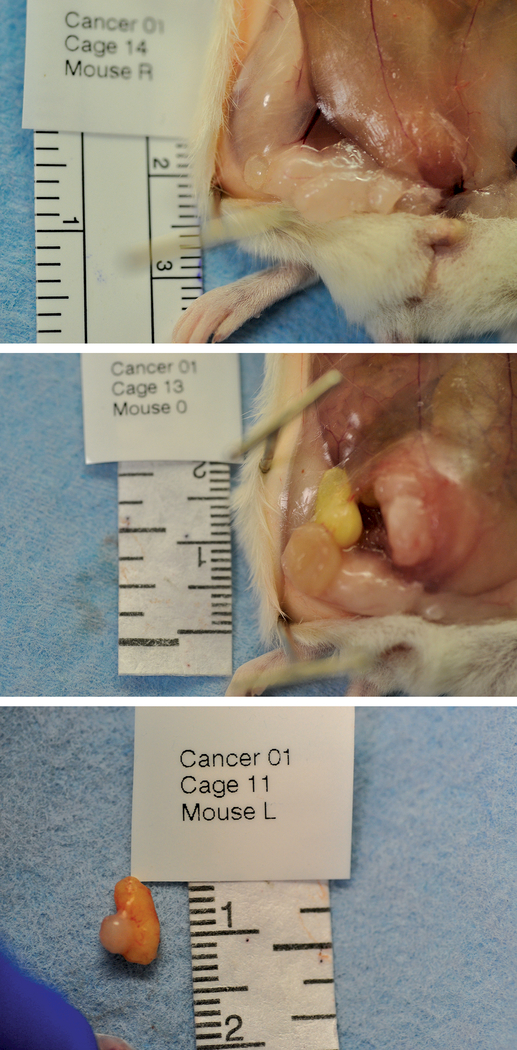
Two weeks after MCF7 xenografting, animals were divided into two groups, with 20 animals receiving 100μl of 0.9% sterile saline injection (Saline group) and 20 animals receiving 100μl of human lipoaspirate (Lipo group). To perform injections, animals were anesthetized, skin was prepared with alcohol and punctured with a 16G beveled needle. Incisions were sealed with Vetbond™ (3M™, Saint Paul, MN, USA). Adipose tissue was prepared prior to injection using the Coleman technique23. Particulated human abdominal tissue was collected through a multi-perforated 3mm blunt cannula connected to 10ml syringes, washed with saline, and collected with centrifugation at 1260g for 3 minutes. Adipose tissue particles were then distributed into 1ml Luer-Lok™ (Becton Dickinson™, Franklin Lakes, NJ, USA) syringes. Xenografts and fat graft procedures are illustrated in figure 2.
Figure 2:
Macroscopic aspect of tumor at right inguinal site from a Saline group animal, 8 weeks after xenograft, demonstrating intimate location of tumor within mammary fat pad, found in 100% tumors in this group (a). Macroscopic aspect of tumors and fat graft at right inguinal site from a Lipo group animal, 8 weeks after xenograft showing tumor and fat graft colocated with mammary fat pads, which was detected in 100% of subjects in this group (b); sample of tumor and fat graft after resection, illustrating deep adherence and the absence of a clear dissection plane between them, which happened in 43% of fat grafts (c).
Abdominal adipose tissue was obtained from a 57 year-old female (BMI of 28kg/m2) during elective abdominal dermoliopectomy. This procedure was done in accordance with an exemption granted by University of Pittsburgh Institutional Review Board (#0511186) for collecting discarded human tissue, without need for consent to participate in the study as long as no patient data or identifiable personal information was obtained. There was no involvement of human as subjects in the experiments.
Macroscopic tumor evaluation
Animals were euthanized 8 weeks after fat graft or saline injection and immediately necropsied. Tumors and adipose tissue were dissected, photographed, measured with a millimeter ruler, weighed in a high precision scale (Sartorius Weighing Technology™, Goettingen, Germany) and measured for volume using a gas pycnometer (AccuPyc™ II 1340, Micrometrics, Norcross, GA, USA). Spleen, liver and lungs were dissected and screened for macroscopic metastasis. Human adipose tissue was dissected and assessed for tissue integrity. Both tumors and adipose tissue were preserved in formalin and histologically assessed.
Histological evaluation
Slides from tumor samples were prepared for hematoxylin-eosin (H&E) and human specific Ki67 (Clone MIB-1, Dako™, Carpenteria, CA, USA). Ki67 is a nuclear protein that is expressed in replicating cells and is often used to calculate a proliferation index in breast tumor. Transplanted adipose tissue viability was assessed using positive expression of perilipin and H&E was used to observe general tissue architecture. Spleen, liver and lungs were assessed with H&E as well.
For H&E staining, samples were deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol solutions using standard histology protocol and incubated in Weighert’s Hematoxylin for 15 minutes. Samples were then differentiated in an HCl-ethanol solution for 1 minute, incubated in Scott’s tap water for 2 minutes and in alcoholic eosin for 2 minutes. Finally, samples were dehydrated, mounted with Permount™ (ThermoFisher Scientific™, Grand Island, NY, USA) and dried overnight.
To stain for Ki67, slides were deparaffinized and rehydrated using standard protocol. Antigen retrieval was performed with Diva Decloaker™ (Biocare Medical™, Concord, CA, USA) retrieval solution pH 6.2 and a decloaking chamber at 120°C. The slides were stained with an Autostainer Plus™ (Dako™) with Tris-buffered saline and Tween 20 rinse buffer (Dako™). Ki-67 antibody was applied at 1:100 dilution. The secondary antibody consisted of Envision Dual Link + (Dako™) HRP polymer. The substrate used was 3,3, Diaminobenzidine + (Dako™). Lastly, the slides were counterstained with Hematoxylin (Dako™), dehydrated and mounted with Permount™.
For perilipin immunohistochemisty, slides were deparrafinized, rehydrated and treated in citrate buffer retrieval solution pH 6 at 100°C for antigen retrieval. Slides were washed with Tris-buffered saline and Tween 20 rinse buffer (Dako™) and Sudan Black B was applied to reduce tissue autofluorescence. Primary antibody PROGEN Biotechnik GmbH ⋅ (Progen™, Heidelberg, Germany) was used in a 1:50 dilution. The secondary consisted of TRITC anti-guinea pig, in a 1:100 dilution. Lastly, the slides were counterstained with DAPI and mounted with fluorescent mounting media.
Histological assessment of tumors was performed by a blinded breast pathologist: tumor detection, histological grade according to Scarff-Bloom-Richardson23 and Ki67 index; as well as screening of spleen, liver and lungs for metastasis. Fat grafts were studied for fat tissue architecture integrity and vascularization.
Statistics
Data of tumor volume and mass was log transformed for statistical analysis and compared with two-tail Student’s T-test; Ki67 indexes were compared with two tailed T-test; and histological grade scores, with Mann-Whitney test. Significance was p<0.05 and tests were performed in SYSTAT 13 software (Systat Software Inc, Chicago, IL, USA) and SPSS 20 (IBM, Armonk, NY, USA).
RESULTS
Animal Mortality
At 72 hours after intervention, one animal receiving lipoaspirate died, however no association with surgical procedure was identified. No other adverse events occurred.
Necropsy findings
During necropsy, all sites injected with cancer cells had macroscopic tumors evident in mammary fat pads 8 weeks after xenografts (Figure 2a). No enlarged lymph nodes were found in axillary or groin regions. No macroscopic metastasis were identified on liver, lungs or spleen.
Macroscopically identifiable adipose tissue was present in all fat graft sites 6 weeks after fat injections (Figure 2b). Average volume retention was 62.7% (+/− 22.3%). Adipose tissue was co-located with tumors in 100% sites and in 43% sites, they were strongly adherent to tumors (Figure 2c).
Macroscopic tumor invasion in neighboring muscle, skin and peritoneum was observed in both saline and Lipo groups, however histological analysis of invasion was not performed as it would interfere with tumor volume measurements.
Table 1 shows the measured tumor mass and volume in lipo and saline groups expressed as mean +/− standard error of the mean. The volume (0.075 +/− 0.010cm3) and mass (0.083 +/− 0.011g) of tumors in Lipo mice were significantly smaller than volume (0.085 +/− 0.008cm3) and mass (0.098 +/− 0.009g) in saline controls (p= 0.046 for volume, and p=0.038 for mass).
Table 1:
Tumors volume and mass in eight weeks
| Group | Tumor volume (cm3) | Tumor mass (g) | P value |
|---|---|---|---|
| Saline | 0.085 (+/−0.008) | 0.098 (+/−0.009) | 0.038* |
| Lipo | 0.075 (+/−0.010) | 0.083 (+/−0.011) | 0.046* |
Average volume in centimeter cube and mass in grams with respective standard error of the mean in Saline and Lipo groups and two-tailed t-test p value.
statistical significance (p<0.05).
Histology
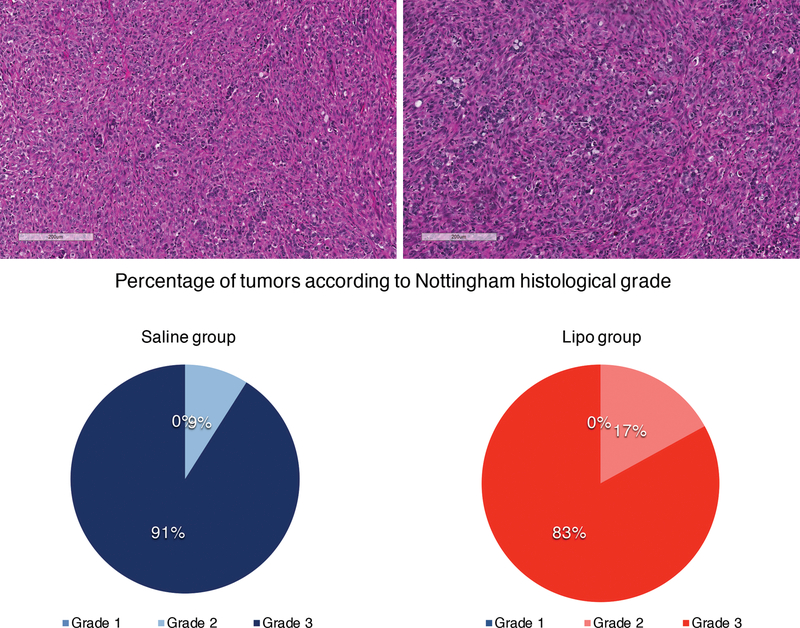
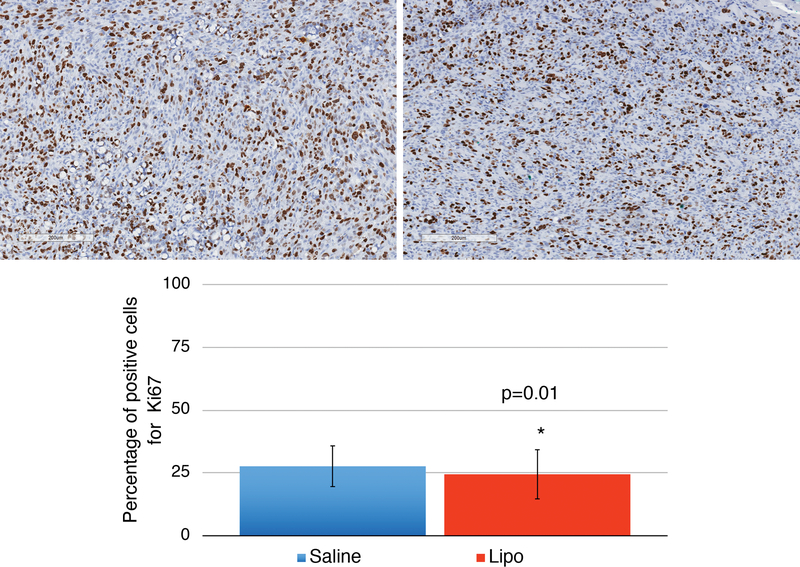
The histological grade and percentage of ki67 positive cells were calculated for tumors from both lipo and saline mice. No difference in histological grade (Figure 3) was detected (p=0.17) and significantly lower ki67 index was found in Lipo animals (p=0.01) (Figure 4). Analysis of adipose tissue architecture suggested that tissue architecture was normal in all samples with few oil cysts and a typical vascular pattern. Adipocytes within grafts were positive for perilipin expression suggesting adipocytes were viable (Figure 5). Liver, lung and spleen were histologically screened for metastasis. No metastatic lesion was detected in any animal (Figure 6).
Figure 3:
Histological grade assessment. HE slides were prepared for Saline group tumors (a) and Lipo group tumors (b) and a histological score was given to each of them based on tubular/glandular differentiation, nuclear pleomorphism and mitotic count (according to Bloom & Richardson, 1957). Scale bars represent 200μm. Percentage of tumors according to histological grade (1, 2 or 3) based on Bloom & Richardson scores in Saline and Lipo groups (c). The majority of tumors in both groups were grade 3 and there was no difference between groups in terms of histological grade (p=0.17).
Figure 4:
Proliferative index. Immune-histochemistry for Ki67 in a Saline group tumor (a) and a Lipo group tumor (b), both highly proliferative. Scale bars represent 200μm. Percentage of Ki67 positive cells in Saline and Lipo group (c). Lower proliferation index was demonstrated in tumors submitted to adjacent fat graft (p=0.01). Error bars represent standard deviation of the mean.
Figure 5:
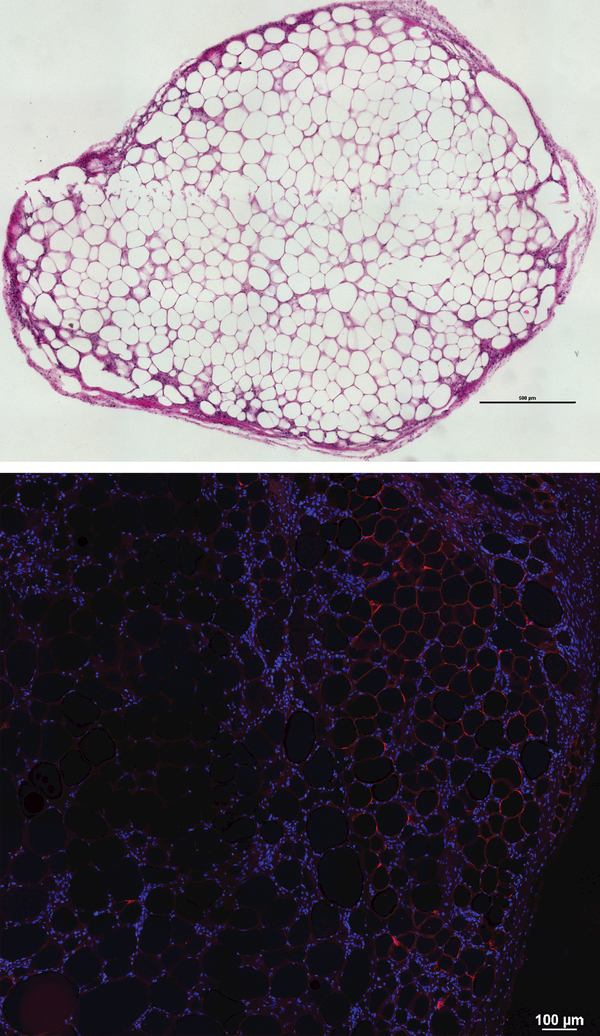
Fat grafts were collected during necropsy and were histologically evaluated. HE demonstrates preserved fat architecture. Scale bar represents 500μm (a). Perilipin staining shows fluorescence in the inner layer of lipid droplets inside adipocytes, confirming the presence of metabolically active adipose tissue in fat grafts after 8 weeks. Scale bar represents 100μm (b).
Figure 6:
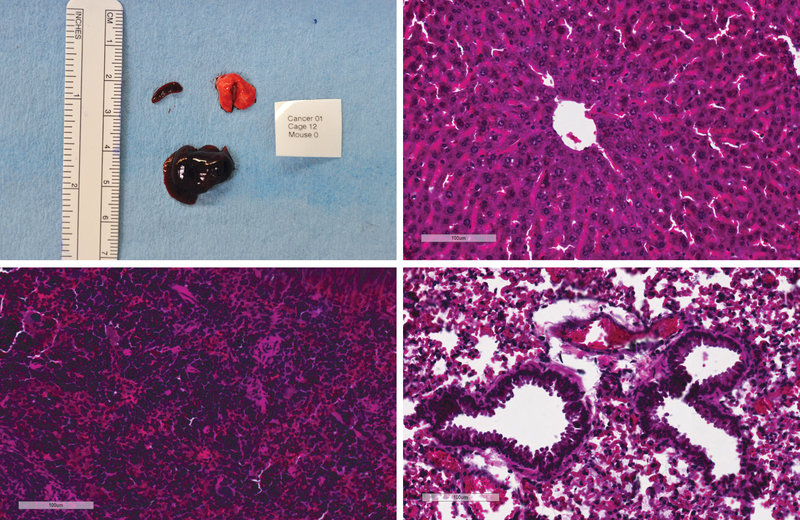
Metastasis screening. Macroscopic aspect of liver, lung and spleen specimens collected from one animal during necropsy. No macroscopic metastasis was identified (a). Histological screening for metastasis was performed on liver (b); spleen (c); and lung (d). No microscopic metastasis was identified in HE slides. Ruler on macroscopic picture correspond to centimeters. Scale bars on HE microscopic pictures correspond to 100um.
DISCUSSION
Although the number of studies on fat grafting alone or in conjunction with flaps or implants for post-oncologic breast reconstruction have increased 60-fold over the past 20 years, approximately 50% of American plastic surgeons have expressed concern that the lack of evidence of fat graft safety is an obstacle for procedure use5–9. Most reported clinical studies primarily including case series or retrospective cohorts, and suggest that fat grafting doesn’t increase local recurrence, or metastatic spread7–14.
Current experimental evidence is discordant with clinical outcomes and data from several in vitro studies suggest that components of adipose tissue increase tumor cell growth, and thus when extrapolated, suggest that perhaps tumor formation or recurrence would also be increased in vivo25. From such studies, authors conclude that secretory factors from adipose tissue may interact with tumor cells and result in increased tumor invasiveness, metastatic potential and expression of genes related to malignancy13,18,21,26–34. These secretory factors are known as adipokines include adiponectin, leptin, resistin, metalloproteinase 11, hepatocyte derived growth factor, collagen VI, interleukins 6 and 8, transforming growth factor alpha and beta, platelet derived growth factor, vascular endothelial growth factor and stromal cell derived factor 1, all with both paracrine and endocrine effects17,18,20,21. While in vitro analyses of isolated cells have been instrumental in isolating components of adipose that may impact tumor physiology, a concurrent drawback is that this does not adequately replicate the complex tissue milieu and thus tumor cell behavior in culture may not sufficiently represent the true in vivo scenario.
Several pre-clinical studies include in vivo experiments. However, current animal models have many shortcomings. Typically animals receive ectopic breast cancer xenografts, preventing tumors from growing within the breast microenvironment; breast cancer cells are grafted at the same time as adipose-derived stem cells, which models the development of a primary breast tumor in the presence of fat tissue; and a high number of breast cancer cells and adipose-derived stem cells (such as 1×106) are studied28–33. Therefore, current in vivo experimental models are far from the very frequent clinical scenario of a breast cancer patient undergoing breast conserving surgery and breast reconstruction with fat grafting. New clinically relevant animal models should provide additional information to correlate experimental in vitro and clinical studies.
A recent study from our group proposed an experimental model of local breast recurrence in the setting of autologous fat grafting for breast reconstruction. Both subcutaneous coinjections and mammary fat pad injections of breast cancer cells and fat graft with an interval of 2 weeks were performed. Cell lines used were highly proliferative triple negative MDA MB 231 and slow growing luminal B like, HER2 positive, BT 474. A very small number of cancer cells (10–104 for MDA MB 231 and 10–106 for BT 474) was injected. Only MDA MB 231 formed tumors in the coinjections with fat graft, but presented lower proliferation. When fat graft was injected 2 weeks after cancer cells, MDA MB 231 had reduced proliferation and increased fibrosis22.
This previous study was the first to point that fat grafting might reduce cancer proliferation in an experimental model in mice. In the present study, the MCF-7 cell line, derived from human breast adenocarcinoma, was used to model residual breast cancer in a mouse mammary pad. This cell line was chosen because of epithelial morphology and hormone (estrogen and progesterone) receptor similarity to luminal A cancer, the most common type of breast cancer35–37. However, this also presents a study limitation in that the cell line is immortalized, highly proliferative and more invasive than tumor isolates, and thus does not reproduce tumor heterogeneity and dormant tumor cells. To mitigate this limitation, a very small number of tumor cells (1000) was used in this study to replicate residual tumors as opposed to the majority of in vivo models that employ high numbers of breast cancer cells prepared by dilution of cell suspensions38–40. The number of injected cells for this study was carefully measured using cell sorting, as opposed to dilution, which also eliminated cell clumping and non-viable cells. Moreover, in the present study, intervention was whole fat graft opposed to isolated and cultured ASCs, which represent a small cellular subpopulation of fat tissue and might have their secretory profile deeply modified by different micoenvironment input41,42.
Our results suggest that that tumor volume and Ki-67 expression were reduced with fat graft compared to saline controls and we hypothesize that this effect was partially due to mechanical pressure exerted by adipose tissue immediately adjacent to tumors. Solid stress in tumor surroundings, such as the presence of transferred adipose tissue can affect cancer growth both by direct cell compression, reducing proliferation and inducing apoptosis, and by compressing blood and lymphatic vessels, causing tumor hypoxia and dissemination43–44. The fact that mechanical stress around solid tumors reduces proliferation may be related both to smaller tumor volume and to lower Ki67 index in Lipo group. However, a few recent studies suggest the possibility of an additional explanation for it. It’s been demonstrated that some components of adipose tissue, such as mesenchymal stem cells, may have anti-tumorigenic effect, depending on specific paracrine input45.
Histological grade similarity is believed to be expected when dealing with cells lines, once they tend to form homogeneous tumors. Also, the absence of metastatic spread is a common finding in cell lines xenografts, even when they are from metastatic origin46.
As an effort to design a new experimental model, the present study has a few shortcomings. Although MCF7 is the most commonly used breast cancer cell line and best compared to luminal A breast cancer. The use of a single breast cancer cell line was primary and further studies with different cell lines should be performed once the model was stablished. The use of a single donor for fat graft was an attempt to control bias from heterogeneity of fat among different patients and different donor sites, but it represents a limitation and the same model should be tested not only with multiple donors but also with multiple donor sites in future studies. Moreover the model is proposed to represent the setting of post-breast conserving surgery by injecting a small number of cancer cells in mammary fat pads, but not by performing surgeries on animals, which does not induce the same inflammatory and wound healing process as a surgical procedure would.
Due to breast cancer heterogeneity, the search for an ideal experimental model is a constant challenge. The type of study, the hypothesis to be tested and the experimental design determine the superiority of one model over another47. Future studies in lipofiling oncologic safety should be performed in immunocompetent animals as immune cells have a very important role in tumor-stroma and tumor-stem cells signaling. Moreover, primary breast tumors cell extracts should be used instead of cell lines, as the former are highly proliferative and have uniform histological features, hindering demonstration of statistical differences between interventions. Different controls able to exert long-term mechanical effect on tumors should be compared to fat graft, such as fillers, acellular dermal matrix or whole dermis.
CONCLUSION
Fat grafting performed in the setting of residual breast tumor in a clinically relevant animal model did not increase tumor size, mass, proliferation, histological grade or metastatic potential. Indeed tumor size after fat grafting even decreased. These findings support oncologic safety of fat grafting for breast reconstruction after cancer therapy.
ACKNOWLEDGMENTS
Liyong Zhang (University of Pittsburgh) and Lisa Bailey (Hillman Cancer Center) for assisting in animal experiments;
Anthony Green and Damian Grybowski (University of Pittsburgh) for assisting in slides preparation;
National Institute of Health for financial support;
Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) for sponsoring MMAS during one year at University of Pittsburgh.
Footnotes
Financial disclosure: The work presented in this manuscripts was supported in full by the National Cancer Institute, National Institutes of Health (NIH) under project number 5R01CA114246. MMAS had financial support (scholarship) from Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). All authors have no commercial association or financial disclosure.
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Authors contributions
Mayara M A Silva: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript;
Miguel Sabino Neto: study conception and design, analysis and interpretation of data, critical revision of manuscript;
Lauren E Kokai: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript;
Vera S Donnenberg: study conception and design, analysis and interpretation of data;
Jefrey L Fine: analysis and interpretation of data (histology);
Kacey G Marra: study conception and design, analysis and interpretation of data, critical revision of manuscript;
Albert D Donnenberg: study conception and design, analysis and interpretation of data;
J Peter Rubin: study conception and design, analysis and interpretation of data, critical revision of manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures and animal care were performed in accordance with protocol approved by University of Pittsburgh Institutional Animal Care and Committee (protocol #1506677).
Human adipose tissue was obtained in accordance with an exemption granted by University of Pittsburgh Institutional Review Board (#0511186) for collecting discarded human tissue.
References
- 1.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veiga DF, Neto MS, Ferreira LM, et al. Quality of life outcomes after pedicled TRAM flap delayed breast reconstruction. Br J Plast Surg. 2004;57(3):252–257. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Huh JS, Min Y-S, Kim HJ, Park HY, Jung T-D. Physical and functional ability recovery patterns and quality of life after immediate autologous latissimus dorsi breast reconstruction: A 1-year prospective observational study. Plast Reconstr Surg. 2015;136(6):1146–1154. [DOI] [PubMed] [Google Scholar]
- 4.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013August;132(2):201e–209e. [DOI] [PubMed] [Google Scholar]
- 5.Kling RE, Mehrara BJ, Pusic AL, et al. Trends in autologous fat grafting to the breast: a national survey of the American society of plastic surgeons. Plast Reconstr Surg. 2013;132(1):35–46. [DOI] [PubMed] [Google Scholar]
- 6.Skillman J, Hardwicke J, Whisker L, England D. Attitudes of UK breast and plastic surgeons to lipomodelling in breast surgery. Breast. 2013;22(6):1200–1204. [DOI] [PubMed] [Google Scholar]
- 7.Petit J, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. 2011;23(3):582–588. [DOI] [PubMed] [Google Scholar]
- 8.Gale KL, Rakha EA, Ball G, Tan VK, McCulley SJ, Macmillan RD. A case-controlled study of the oncologic safety of fat grafting. Plast Reconstr Surg. 2015;135(5):1263–1275. [DOI] [PubMed] [Google Scholar]
- 9.Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;137(2):385–393. [DOI] [PubMed] [Google Scholar]
- 10.Brenelli F, Rietjens M, De Lorenzi F, et al. Oncological safety of autologous fat grafting after breast conservative treatment: a prospective evaluation. Breast J. 2014;20(2):159–165. [DOI] [PubMed] [Google Scholar]
- 11.Petit J, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intra epithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;24(6):1479–1484. [DOI] [PubMed] [Google Scholar]
- 12.Petit JY, Maisonneuve P, Rotmensz N, Bertolini F, Rietjens M. Fat Grafting after Invasive Breast Cancer: A Matched Case-Control Study. Plast Reconstr Surg. 2017June;139(6):1292–1296; [DOI] [PubMed] [Google Scholar]
- 13.Gutowski KA, Force AFGT. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124(1):272–280. [DOI] [PubMed] [Google Scholar]
- 14.Rigotti G, Marchi A, Stringhini P, et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;34(4):475–480. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Plastic Surgeons Executive Committee. ASPS Guiding Principles: 2012 Post-Mastectomy Fat Graft/Fat Transfer. Available at: www.plasticsurgery.org; Accessed September 4, 2017.
- 16.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119(3):775–785. [DOI] [PubMed] [Google Scholar]
- 17.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163(4):399–408. [DOI] [PubMed] [Google Scholar]
- 18.Nepal S, Kim MJ, Hong JT, et al. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: involvement of p53/FoxO3A axis. Oncotarget. 2015;6(9):7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva MMA, Donenberg VS, Rubin JP, Zimmerlin L, Donnenberg AD. The immune milieu of adipose tissue: implications for autologous lipotransfer and other therapeutic applications. In: Coleman S, Mazzola R, Pu L, eds. Fat injection: from filling to regeneration, 2nd ed. Boca Raton(FL):CRC Press. 2016:200–227; [Google Scholar]
- 20.Schweizer R, Tsuji W, Gorantla VS, Marra KG, Rubin JP, Plock JA. The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem cells Int. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai M, Miki Y, Takagi K, et al. Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment. Breast Cancer Res. 2017June19;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji W, Valentin JE, Marra KG, Donnenberg AD, Donnenberg VS, Rubin JP . An Animal Model of Local Breast Cancer Recurrence in the Setting of Autologous Fat Grafting for Breast Reconstruction. Stem Cells Transl Med. 2018January;7(1):125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman SR. Structural Fat Grafting. Plast Reconstr Surg. 2005;115(6):1777–1778. [Google Scholar]
- 24.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer: a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957September;11(3):359–77; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2010;17(1–2):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielli A, Scioli MG, Gentile P, et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertolini F, Petit J, Kolonin M. Stem cells from adipose tissue and breast cancer: hype, risks and hope. Br J Cancer. 2015;112(3):419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charvet HJ, Orbay H, Wong MS, Sahar DE. The oncologic safety of breast fat grafting and contradictions between basic science and clinical studies: a systematic review of the recent literature. Ann Plast Surg. 2015;75(4):471–479. [DOI] [PubMed] [Google Scholar]
- 29.Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5(3):613–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter M, Liang S, Ghosh S, Hornsby P, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. [DOI] [PubMed] [Google Scholar]
- 32.Welte G, Alt E, Devarajan E, Krishnappa S, Jotzu C, Song YH. Interleukin‐8 derived from local tissue‐resident stromal cells promotes tumor cell invasion. Mol Carcinog. 2012;51(11):861–868. [DOI] [PubMed] [Google Scholar]
- 33.Devarajan E, Song YH, Krishnappa S, Alt E. Epithelial–mesenchymal transition in breast cancer lines is mediated through PDGF‐D released by tissue‐resident stem cells. Int J Cancer. 2012;131(5):1023–1031. [DOI] [PubMed] [Google Scholar]
- 34.Jotzu C, Alt E, Welte G, et al. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Oncol. 2011;34(1):55–67. [DOI] [PubMed] [Google Scholar]
- 35.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J of Clin Oncol. 2010;28(10):1684–1691. [DOI] [PubMed] [Google Scholar]
- 36.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011August12;13(4):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowan BG, Lacayo EA, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of head and neck cancer xenografts. Aesthet Surg J. 2015;36(1):93–104. [DOI] [PubMed] [Google Scholar]
- 39.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17(3):463–474. [DOI] [PubMed] [Google Scholar]
- 40.Kucerova L, Kovacovicova M, Polak S, et al. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 2011;58(5):361–70. [DOI] [PubMed] [Google Scholar]
- 41.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011April1;71(7):2455–65; [DOI] [PubMed] [Google Scholar]
- 42.Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst). 2010;33(2):61–79; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng G, Tse J, Jain RK, Munn LL. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS one. 2009;4(2):e4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stylianopoulos T The solid mechanics of cancer and strategies for improved therapy. J Biomech Eng. 2017;139(2):021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7(9):e45590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdall SE, Hanby AM, Lansdown MRJ, Speirs V: Breast cancer cell lines: friend or foe? Breast Cancer Res 2003, 5:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner KU. Models of breast cancer: quo vadis, animal modeling? Breast Cancer Res. 2004;6(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]