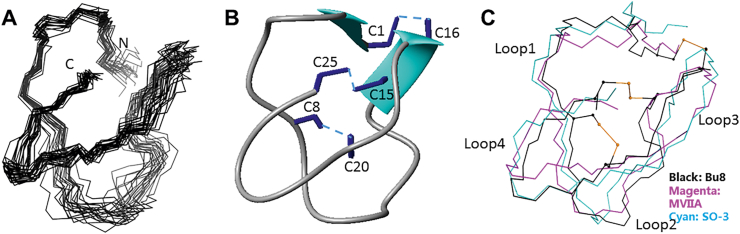
Figure 3.
3D NMR solution structures of Bu8 and its comparisons with MVIIA and SO-3. (A) Superposition of 20 structures of Bu8 with the lowest energy, aligned with residues 2–7 and residues 19–24. The data were submitted to the BMRB database (ID: 36177) and the PDB database (ID 5ZNU). (B) Bu8 backbone with disulfide bonds (C1–C16, C8–C20, and C15–C25) and β-sheet structure. (C) Comparison of the backbone conformations of Bu8 (black), MVIIA (magenta), and SO-3 (cyan). The peptides are divided into loops 1–4, namely residues 2–7, 9–14, 17–19, and 21–24, respectively, interspaced by the cysteines. The toxins are aligned by backbone and the side chains of residues 2–7, 19–24.