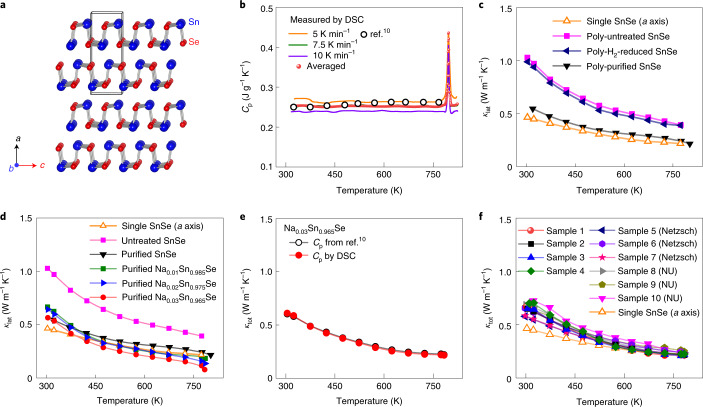
Fig. 4. SnSe crystal structure and lattice, κlat, and total thermal conductivities, κtot, as a function of temperature for the undoped and Na-doped polycrystalline SnSe samples before and after the purification process.
a, Room temperature crystal structure (Pnma space group) viewed down the b axis9: Sn atoms, blue; Se atoms, red. b, Temperature-dependent heat capacity (Cp) measured by DSC for the purified Na0.03Sn0.965Se samples. Orange, green and purple solid lines denote the Cp recorded at the heating rate of 5, 7.5 and 10 K min−1, respectively. The averaged Cp values are represented by red circles, which are used to calculate the κtot. Cp values derived from the previous work are included for comparison (black circles)10. c, κlat for the untreated, H2-reduced without Sn purification and purified SnSe samples. d, κlat for the NaxSn0.995–xSe (x = 0.01, 0.02 and 0.03) samples in comparison with that for the untreated and purified SnSe samples. e, κtot of the Na0.03Sn0.965Se sample calculated using the Cp obtained by our DSC experiments (red circles) and derived from the previous works (black circles)10. f, The reproducibility of κtot for ten independently synthesized samples, cross-checked at SNU (samples 1–4), Netzsch Instruments (Netzsch, samples 5–7) and Northwestern University (NU, samples 8–10). κlat and κtot for a SnSe single crystal along the a axis are given for comparison9 in c,d,f. Polycrystalline samples were measured parallel to the SPS direction.