Abstract
Background
5–10% of amyotrophic lateral sclerosis (ALS) patients presented a positive family history (fALS). More than 30 genes have been identified in association with ALS/frontotemporal dementia (FTD) spectrum, with four major genes accounting for 60–70% of fALS. In this paper, we aimed to assess the contribution to the pathogenesis of major and rare ALS/FTD genes in ALS patients.
Methods
We analyzed ALS and ALS/FTD associated genes by direct sequencing or next-generation sequencing multigene panels in ALS patients.
Results
Genetic abnormalities in ALS major genes included repeated expansions of hexanucleotide in C9orf72 gene (7.3%), mutations in SOD1 (4.9%), FUS (2.1%), and TARDBP (2.4%), whereas variants in rare ALS/FTD genes affected 15.5% of subjects overall, most frequently involving SQSTM1 (3.4%), and CHMP2B (1.9%). We found clustering of variants in ALS major genes in patients with a family history for “pure” ALS, while ALS/FTD related genes mainly occurred in patients with a family history for other neurodegenerative diseases (dementia and/or parkinsonism).
Conclusions
Our data support the presence of two different genetic components underlying ALS pathogenesis, related to the presence of a family history for ALS or other neurodegenerative diseases. Thus, family history may help in optimizing the genetic screening protocol to be applied.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-021-10521-w.
Keywords: Amyotrophic lateral sclerosis, Frontotemporal degeneration, Next generation sequencing, Genetic heterogeneity, Mutation screening
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by the degeneration of upper and lower motor neurons. The risk of developing ALS peaks at 50–75 years of age and decreases subsequently. Survival is highly variable, but respiratory failure usually leads to death about 3–4 years after onset [1]. In up to 50% of cases, there are extra-motor manifestations such as behavior changes, executive dysfunctions, and language problems. In 10–15% of patients, these problems are severe enough to meet the clinical criteria for frontotemporal dementia (FTD) [2]. The combination of FTD with motor neuron disease (MND) refers to ALS/FTD [3]. The relationship between ALS and FTD has been confirmed by genetic studies, and these two conditions are now considered at the opposite extremes of a clinical-pathological continuum [4, 5].
Most cases are sporadic (sALS), but 10–20% of patients present a familial recurrence (fALS) [6], usually showing an autosomal dominant transmission [4]. Recent studies have highlighted the role of genetic risk factors even in sporadic patients, where heritability would represent about 21.0% [7], and twin studies have estimated a heritability of about 60% [8].
More than 30 genes have been linked to ALS and the ALS/FTD continuum [9], in addition to a small percentage of patients with sALS [10]. Four major genes, chromosome 9 open reading frame 72 (C9orf72), superoxide dismutase 1 (SOD1), TAR DNA-binding protein (TARDBP), and fused in sarcoma (FUS) cover up to 60% of fALS and 10–13% of sALS cases [9]. Variants in other genes are found in < 1% of patients [10, 11].
An increasing number of cohort studies have investigated the relationship between genes and clinical phenotypes [12], as a model for researching the mechanisms underlying the disease onset and progression. They demonstrated that incomplete penetrance and genetic pleiotropy further complicate the scenario. Furthermore, more than one potentially pathogenic variant can be identified in a single patient, suggesting an oligogenic inheritance with a dose-dependent gene-burden effect [13, 14].
In this study, we compared the clinical and genetic data of Italian ALS patients to assess the genetic contribution to the pathogenesis of familial and sporadic ALS.
Methods
Patients
We collected clinical and genetic data from patients with definite, probable, probable laboratory-supported, and possible ALS diagnosed according to the revised El Escorial criteria [15] at the Clinica Neurologica, Bellaria Hospital (Bologna, Italy) between 2010 and 2019. Clinical data include gender, age at onset (AAO), type of onset, ALS phenotype, and family history. Regarding family history, we differentiated patients with a positive family history for ALS (fALS-ALS) from patients with a family history for other neurodegenerative diseases (fALS-ND), i.e., dementia and parkinsonism. Patients without a family history for ALS, parkinsonism or dementia were defined as sporadic (sALS).
Patients were classified into the following clinical phenotypes: classic, bulbar, predominant upper motor neuron (PUMN), and predominant lower motor neuron (PLMN) [16, 17]. Due to the relative small sample, we decided to include patients with flail arm, flail leg, and progressive muscular atrophy (PMA) variants in the PLMN group, while patients with primary lateral sclerosis were included in the PUMN category. Cognitive status was assessed through the clinical history, neurological examination, and a neuropsychological assessment including the Frontal assessment battery [18], and the Brief Mental Deterioration Battery (BMDB) [19].
For possible ALS, probable laboratory-supported ALS and probable ALS the diagnosis was confirmed during follow-up visits. The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines, and approved by the local ethics committee “Area Vasta Emilia Centro”. Informed consent was given by participants.
Genetic analysis
DNA extraction
Genomic DNA (gDNA) was extracted from peripheral blood by standard procedures [13]. gDNA was quantified using the Quantus Fluorometer (Promega) with QuantiFluor double-stranded DNA system (Promega).
Gene sequencing
All 330 patients were screened for mutations in ALS major genes [20]: SOD1 (all exons), FUS (exons 6 and 15), TARDBP (exons 2, 3, and 5) genes and for pathogenic repeat expansion (RE) in the C9orf72 gene as previously reported [13, 21].
Since 2015, genetic screening has been performed by next generation sequencing (NGS) multigene panels. We used one of the following custom gene panels: amplicon-based Illumina panel (TruSeq Custom Amplicon 1.5, Illumina, CA, USA) [22]; probe-based Illumina panel (Nextera Rapid Capture, Illumina); probe-based Agilent panel (Sure Select XT2, Agilent Technologies, Santa Clara, CA, USA), and Neurodegeneration Illumina panel (TruSeq Neurodegeneration, Illumina). For the purpose of this study, we analyzed only genes previously linked to ALS or ALS/FTD spectrum: CCNF, CHCHD10, CHMP2B, DCTN1, FIG4, GRN, MAPT, OPTN, SETX, SQSTM1, TBK1, TREM2, TYROBP, UBQLN2, and VCP (Table S1). Due to the presence of patients with a family history of dementia, we also searched for rare pathogenic variants in genes linked to other forms of dementia: APP, PSEN1, PSEN2, ITM2B, CSF1R, and NOTCH3 genes (Table S1). Rare ALS genes (ALS2, ANG, PFN1, SPAST, TUBA4A, UBQLN1, and VAPB), and ALS risk factor genes (NEFH, NEK1, and C21orf2) were analyzed by Neurodegeneration Illumina panels in 51 patients (Table S1).
Enriched libraries were sequenced using MiSeq or NextSeq sequencer (Illumina), with a paired-end approach. Data were automatically analyzed by MiSeq Reporter software (Illumina) on the instrument, or by an in-house bioinformatic pipeline (Supplementary Materials). Variants were reported using the HGVS-Sequence Variant Nomenclature.
Variant classification
Variant selection was performed with BaseSpace Variant interpreter (Illumina, CA, USA). Rare single-nucleotide variant (SNV) and small indels with a minor allele frequency (MAF) < 1% in the 1000 Genome Project database (http://browser.1000genomes.org/) or in the Genome Aggregation Database (GnomAD) [23] were selected. Variants were classified according to the American College of Medical Genetics and Genomics guidance for the interpretation of sequence variants [24]. Those reported in the ClinVar or HGMD (The Human Gene Mutation Database) databases were classified accordingly as known disease-causing variant (pathogenic) or variant of uncertain significance (VUS). Novel variants’ pathogenicity was assessed with in-silico effect predictor tools (Supplementary Materials).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 25 (IBM, Armonk, NY, USA). Kolmogorov–Smirnov test was used to verify the normal distribution of the data. Quantitative continuous variables were described as mean and standard deviation (SD) if the distribution was normal, or as median and range otherwise. Categorical variables were expressed as counts and percentages.
For continuous variables, the Mann–Whitney U or the Kruskal–Wallis tests were used to test differences between two or more groups, respectively. The chi-squared test was adopted for categorical variables and the post hoc test with Bonferroni adjustment was used if the overall chi-squared test was significant. For statistical tests, p < 0.05 was considered significant.
Results
Clinical features
A total of 330 Italian patients were included in the study and their clinical data are shown in Table 1. The median AAO was 63 years, ranging from 27 to 87 years (Table 1).
Table 1.
Clinical features of study population
| Patients/clinical characteristics | N (tot. 330) | % |
|---|---|---|
| Gender | ||
| Male | 173 | 52.4 |
| Female | 157 | 47.6 |
| Age at onset (y) | ||
| Median (range) | 63 (27–87) | |
| Type of onset | ||
| Bulbar | 95 | 28.8 |
| Spinal | 193 | 58.5 |
| Pseudo-polyneuritic | 18 | 5.5 |
| Pyramidal | 24 | 7.3 |
| ALS variant | ||
| Classic | 244 | 74.8 |
| Bulbar | 17 | 5.2 |
| PLMN | 30 | 10.9 |
| PUMN | 39 | 9.1 |
| Deceased patients | 187 | 56.7 |
| Disease duration (m) | ||
| Median (range) | 35 (4–169) | |
| Family history | ||
| fALS | 84 | 25.5 |
| fALS-ALS | 30 | 9.1 |
| fALS-ND | 54 | 16.4 |
| sALS/unknown | 222 | 74.5 |
| Other clinical features | ||
| Dementia | 21 | 6.4 |
ALS amyotrophic lateral sclerosis; fALS familial ALS; fALS-ALS familial ALS with positive family history for ALS; fALS-ND familial ALS with positive family history for other neurodegenerative diseases; m months; N number; PLMN predominant lower motor neuron; PUMN predominant upper motor neuron; sALS sporadic ALS
Overall, ALS phenotypes were distributed as follows: bulbar 5.2%, classic 74.8%, PLMN 10.9%, and PUMN 9.1%; 6.4% of all patients also showed cognitive deficits, mainly consistent with FTD. Our cohort includes 20.9% of patients with early-onset, defined as young-onset ALS (before or at the age of 50, arbitrary cut-off [25]). These patients showed a significantly higher percentage of PUMN variants than the other patients (15.9% vs. 7.3%, p value = 0.017).
Analysis of family history showed that 9.1% of our patients had a positive family history for ALS (fALS-ALS) (Table 1). Given the ALS/FTD continuum, we examined the percentage of patients with relatives affected by other neurodegenerative disorders, particularly dementia and/or parkinsonism (fALS-ND). These patients accounted for 16.4% of the cohort. Thus, 25.5% of our patients had a familial form of the ALS/FTD continuum [26]. Overall, fALS patients had a significantly earlier AAO (median 59 years) than sALS (median 64 years), p value = 0.047.
Stratifying the familiarity for AAO, we observed a progressive decrease in familial forms after age 60 (patients with AAO before 60 had a familial form in 34.4% of cases compared to 22.3% of patients with AAO after age 60, p value = 0.034; Fig. 1a). However, the trend was different between fALS-ALS and fALS-ND subgroups (Fig. 1b). Indeed, the percentage of fALS-ALS was higher in patients with young-onset ALS (18.8% of ALS patients with AAO ≤ 50 as compared to 6.5% of patients with AAO > 50, p value = 0.007), while no differences were observed concerning the family history of fALS-ND.
Fig. 1.
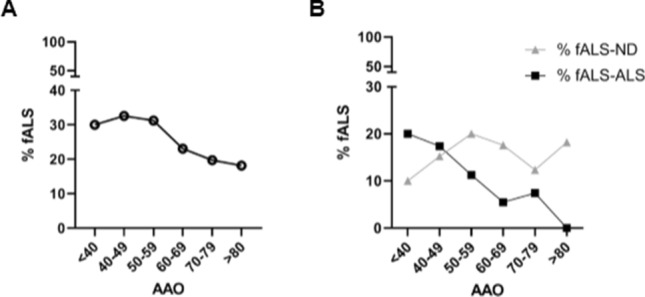
Inheritance features of the study population. A, b The graph shows the percentage of ALS patients with positive family history (a, fALS); for ALS (b, fALS-ALS) and for other neurodegenerative diseases (b, fALS-ND) stratified for age at onset (AAO)
Patients with cognitive decline showed a high percentage of positive family history for ALS and/or dementia/parkinsonism (55.5% vs 25.5%, p value = 0.004), while they did not significantly differ for AAO (p value = 0.558). Family history was similar among the different ALS phenotypic variants.
Genetic screening
All patients were screened for the RE in the C9orf72 gene and for mutations in SOD1, FUS, and TARDBP. 207 patients, enriched for a positive family history, were also analyzed by NGS multigene panels to verify the presence of mutations in the rare genes associated with ALS or the ALS/FTD continuum, and other dementia genes. 51 patients were also tested with a more extensive panel to test the genes reported as risk factors in ALS and other rare genes (Table S1).
Genetic variants in the ALS major genes
Fifty-five patients (16.7%) presented a pathogenic or probably pathogenic genetic variant in one of the four major genes (Table 2). The mutation frequency was 41.7% in fALS and 8.1% in sALS. These frequencies were significantly different between fALS-ALS and fALS-ND patients (76.7% vs. 22.2%, p value < 0.0001).
Table 2.
Number of pathogenic mutations in ALS-major genes identified in this study
| Gene | All (n = 330) | fALS (n = 84) | sALS (n = 246) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | fALS-ALS (n = 30) | fALS-ND (n = 54) | n | % | |||
| N | % | n | % | |||||||
| C9orf72 | 24 | 7.3 | 18 | 21.4 | 8 | 26.7 | 10 | 18.5 | 6 | 2.4 |
| SOD1 | 16 | 4.9 | 8 | 9.5 | 8 | 26.7 | 0 | 0.00 | 8 | 3.3 |
| FUS | 7 | 2.1 | 4 | 4.8 | 3 | 10.00 | 1 | 1.9 | 3 | 1.2 |
| TARDBP | 8 | 2.4 | 5 | 6.0 | 4 | 13.3 | 1 | 1.9 | 3 | 1.2 |
| Total | 55 | 16.7 | 35 | 41.7 | 23 | 76.7 | 12 | 22.2 | 20 | 8.1 |
ALS amyotrophic lateral sclerosis; fALS familial ALS; fALS-ALS familial ALS with positive family history for ALS; fALS-ND familial ALS with positive family history for other neurodegenerative diseases; n number; sALS sporadic ALS
The pathogenic RE in the C9orf72 gene was the most frequent mutation (n = 24, 7.3% of all cases, Table 2). Among these, 18 patients (21.4% of fALS) presented a positive family history, with no significant difference between the percentages in two subgroups of fALS, 26.7% among fALS-ALS cases, and 18.5% among fALS-ND cases (p value = 0.55). Six patients were sALS (2.4% of all sALS cases). In one patient, we identified an intermediate repeat expansion (24–30 repeats) [27].
Clinical features of C9orf72 RE carriers are detailed in Table S2. We then identified 11 different variants in the SOD1 gene (Tables 2 and S3), of which five classified as definite pathogenic, five as VUS, and one (c.73-4A > G), never reported, which was predicted to be likely benign (Table S4). All variants classified as VUS on HGMD or ClinVar were considered as likely causative [28–31] since for several SOD1 variants a reduced penetrance has been described and they can, therefore, rarely be present in controls [32]. The SOD1 variants represented the second genetic cause in our cohort (n = 16, 4.9% of all cases, Table 2). Eight patients were fALS, all of them with a family history of ALS (26.7% of fALS-ALS) and the remaining eight were sALS (3.3% of sALS) (Table 2).
Two novel mutations were identified in the FUS gene (Table S3). Both were predicted to be damaging (Tables S3 and S4). Five variants, previously described, were identified in additional five patients (Table S3). Thus, mutations in FUS explained 2.1% of all cases, 4.8% of fALS (10.0% fALS-ALS and 1.9% fALS-ND), and 1.2% sALS (Table 2).
Eight patients carried mutations in the TARDBP gene (Table 2 and S3). Variant p.Gln303His was considered likely pathogenic because it was previously described in a sALS [33]. Overall, mutations in the TARDBP gene were identified in 2.4% of our cohort, involving 6.0% of fALS (13.3% of fALS-ALS, and 1.9% of fALS-ND, p value < 0.0001), and 1.2% of sALS (Table 2).
Genetic variants identified by NGS analysis
Among the 207 patients tested for mutations in genes rarely involved in ALS, ALS/FTD continuum, or other types of degenerative dementia (Table S1), 31 patients (14.97% of the cohort) carried pathogenic or likely pathogenic variants (Table 2). Of the 29 different variants identified, 13 were previously reported in public databases (ClinVar or HGMD) or in the literature (Table 3). Only one variant (OPTN p.Gln314Leu) was reported as definite pathogenic; the others were classified as VUS. In addition, we identified 16 new variants considered potentially pathogenic based on the in-silico prediction of pathogenicity (Tables S4 and S5).
Table 3.
Clinical features of FTD/ALS continuum genes mutations carriers
| ID patients | Gene | Variant | Classification [REF] | Gender | Family history | AAO | Variant of ALS | Additional clinical signs | Disease duration (m) | Other mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| ALS#299 | SQSTM1 | c.86C > G p.Pro29Arg | R-VUSa,b | M | fALS-ND | 62 | Classic | > 52 | ||
| ALS#298 | c.98C > T p.Ala33Val | R-VUSa,b [51] | F | sALS | 46 | Classic | > 26 | |||
| ALS#261 | c.241G > A p.Glu81Lys | R-VUSa,b | F | fALS-ND | 78 | Classic | 40 | |||
| ALS#317 | c.481C > T p.Arg161Trp | R-VUSa | M | sALS | 62 | Classic | > 41 | |||
| ALS#212 | c.962G > A p.Arg321His | R-VUSa,b | M | sALS | 70 | Classic | 54 | |||
| ALS#124 | c.1175C > T p.Pro392Leu | R-VUSa,b | M | sALS | 38 | Classic | > 10 | |||
| ALS#23 | c.1175C > T p.Pro392Leu | R-VUSa,b | M | sALS | 42 | Classic | 22 | |||
| ALS#192 | APP | c.2212G > A p.Val738Ile | N-VUS | F | sALS | 40 | PUMN | 123 | ||
| ALS#159 | CCNF | c.656 T > C p.Leu219Pro | N-likely pathogenic | M | fALS-ND | 61 | Classic | 76 | ||
| ALS#126 | CHCHD10 | c.42-2A > C | N-likely pathogenic | F | sALS | 45 | Classic | > 40 | NOTCH3 | |
| ALS#208 | c.100C > T p.Pro34Ser | R-VUSa,b | M | sALS | 73 | Classic | Cognitive deficits | 53 | ||
| ALS#18 | CHMP2B | c.85A > G p.Ile29Val | R-VUSa,b | M | sALS | 64 | Classic | 64 | ||
| ALS#157 | c.85A > G p.Ile29Val | R-VUSa,b | M | fALS-ND | 65 | Classic | > 29 | TARDBP | ||
| ALS#85 | c.142A > C p.Lys48Gln | N-likely pathogenic | F | sALS | 72 | Classic | 18 | |||
| ALS#225 | c.557G > A p.Arg186Gln | N-likely pathogenic | F | fALS-ND | 54 | Classic | 11 | |||
| ALS#65 | CSF1R | c.1420G > A p.Val474Ile | R-VUSa | M | fALS-ND | 67 | PUMN | > 110 | ||
| ALS#283 | DCTN1 | c.71C > T p.Ala24Val | N-likely pathogenic | M | sALS | 38 | Classic | > 73 | ||
| ALS#181 | c.71C > T p.Ala24Val | N-likely pathogenic | M | sALS | 50 | Classic | > 30 | |||
| ALS#325 | FIG4 | c.1424G > A p.Gly475Asp | N-likely pathogenic | M | fALS-ND | 47 | Bulbar | > 9 | ||
| ALS#101 | c.434C > T p.Pro145Leu | N-likely pathogenic | F | sALS | 87 | Classic | 24 | |||
| ALS#328 | ITM2B | c.511A > C p.Thr171Pro | N-likely pathogenic | F | sALS | 60 | Classic | > 4 | ||
| ALS#141 | c.751A > G p.Ile251Val | R-VUS [52] | M | fALS | 45 | PUMN | Optic atrophy, deafness, parkinsonism, dystonia, schizophrenia | 72 | C9orf72 | |
| ALS#126 | NOTCH3 | c.2203C > T p.Arg735Ter | N-likely pathogenic | F | sALS | 45 | Classic | > 40 | CHCHD10 | |
| ALS#273 | c.2618G > A p.Cys873Tyr | N-likely pathogenic | F | fALS-ND | 57 | Classic | > 29 | |||
| ALS#114 | OPTN | c.235C > T p.Gln79Ter | N-likely pathogenic | F | sALS | 80 | Classic | 24 | ||
| ALS#82 | c.941A > T p.Gln314Leu | R-pathogenica,b | F | sALS | 42 | Spinal | 48 | SOD1 | ||
| ALS#230 | SETX | c.430A > G p.Asn144Asp | N-likely pathogenic | F | sALS | 50 | Classic | > 55 | ||
| ALS#74 | c.1255A > G p.Met419Val | N-likely pathogenic | M | sALS | 72 | Classic | 5 | |||
| ALS#135 | c.4982C > G p.Pro1661Arg | R-VUSa | F | sALS | 51 | Classic | 113 | SOD1 | ||
| ALS#215 | TBK1 | c.225G > C p.Glu75Asp | N-likely pathogenic | F | sALS | 61 | Classic Cognitive deficits | > 37 | ||
| ALS#239 | c.802A > G p.Ser268Gly | N-likely pathogenic | F | fALS-ND | 65 | Classic | > 19 | |||
| ALS#161 | UBQLN1 | c.162G > T p.Glu54Asp | R-VUSa,b | M | fALS-ND | 59 | Classic | > 109 | ||
Only patients who carried pathogenic/likely pathogenic variants have been reported (pathogenic prediction of novel variants are shown in Tables S4 and S5). The clinical characteristics of patients who carried novel variants predicted as likely benign are in Table S7
AAO age at onset; ALS amyotrophic lateral sclerosis; fALS familial ALS, fALS-ALS familial ALS with positive family history for ALS; fALS-ND familial ALS with positive family history for other neurodegenerative diseases; m months; N novel variant; PLMN predominant lower motor neuron; PUMN predominant upper motor neuron; R reported variant; REF reference; VUS variant of uncertain significance
aVariants reported in ClinVar
bVariants reported in HGMD
The genes in which we identified more variants were SQSTM1 (n = 7, 3.4% of the cohort) and CHMP2B (n = 4, 1.9%). We also identified variants in: CHCHD10 (n = 2), CCNF (n = 1), DCTN1 (n = 2), FIG4 (n = 2), OPTN (n = 2), SETX (n = 3), TBK1 (n = 2), and UBQLN1 (n = 1).
Finally, we identified additional rare variants in non-FTD dementia related genes such as APP, CSF1R, ITM2B, and NOTCH3 (Table 3). All patients carrying variants in APP, CSF1R, ITM2B (p.Ile251Val), and NOTCH3 genes showed a family history for dementia.
Four patients also carried additional pathogenic variants in one of the ALS-major genes (Table 3).
Comprehensive analysis of the ALS mutation spectrum with an extended panel
To assess genetic variants in additional ALS-related genes (PFN1, ANG, NEFH, NEK1, TUBA4A, C21orf2, and SPAST) (Table S1) a subgroup of patients (n = 51) was screened with an extended NGS panel. Rare potentially pathogenic variants were identified in eight patients (Table 4).
Table 4.
Genetic variants identified in ALS risk factor genes
| ID Patients | Gene (no of patients) | Variant | Classification [REF] | Gender | Family history | AAO | Variant of ALS | Additional clinical signs | Disease duration (m) | Other mutations (gene) |
|---|---|---|---|---|---|---|---|---|---|---|
| ALS#77 | ALS2 | c.3206G > A p.Gly1069Glu | VUS | M | fALS-ND | 63 | Classic | 15 | C9Orf72 | |
| ALS#86 | ALS2 | c.3128G > A p.Arg1043His | N-likely pathogenic | F | sALS | 76 | PUMN | 36 | ||
| ALS#264 | ALS2 | c.1115C > G p.Pro372Arg | VUS | F | fALS-ND | 62 | Classic | 119 | ||
| ALS#10 | NEK1 | c.3793C > T p.His1265Tyr | VUS | M | sALS | 63 | Classic | 50 | ||
| ALS#72 | NEK1 | c.686A > G p.Tyr229Cys | VUS | F | sALS | 73 | Classic | > 23 | SPAST | |
| ALS#152 | NEK1 | c.1535C > T p.Ala512Val | VUS | F | sALS | 41 | Classic | 133 | ||
| ALS#23 | SPAST | c.1625A > G p.Asp542Gly | VUS [53] | M | sALS | 38 | Classic | > 10 | SQSTM1 | |
| ALS#72 | SPAST | c.98C > T p.Pro33Leu | VUS | F | sALS | 73 | Classic | > 23 | NEK1 |
Only patients who carried pathogenic/likely pathogenic variants have been reported (pathogenic prediction of novel variants are shown in Tables S5)
AAO age at onset; ALS amyotrophic lateral sclerosis; fALS familial ALS; fALS-ND familial ALS with positive family history for other neurodegenerative diseases; m months; N novel variant; PLMN predominant lower motor neuron; PUMN predominant upper motor neuron; REF reference; VUS variant of uncertain significance
In particular, we found a variant in the NEK1 gene in three patients, three heterozygous variants in the ALS2 (n = 3), and two variants in the SPAST gene, rarely linked to ALS [34]. One patient carried variants in two rare genes (NEK1 and SPAST genes), two an additional pathogenic variant in SQSTM1, and C9orf72 genes (Table 4). No mutations have been identified in PFN1, ANG, NEFH, and TUBA4A genes.
Genotype–phenotype correlation
The average AAO and disease duration in patients carrying variants in the four major ALS genes, and in ALS-rare genes are shown in Table S6.
SQSTM1 was the most frequent gene carrying potentially pathogenic variants among the rare ALS genes explored (Table 3). Thus, to characterize a possible correlation with a specific phenotype, we considered this gene separately from other ALS-rare genes.
Although we did not find any significant difference between genetic groups, patients with SQSTM1 mutations presented with a mean AAO lower and more similar to that of four major ALS genes, while patients carrying rare ALS/FTD genes mutations showed an AAO comparable to that of wild-type patients. Concerning the phenotype associated with the different variants, TARDBP patients showed a bulbar phenotype more frequently than wild-type patients (37.5% vs. 3.6%, p value = 0.0001), while FUS mutated patients showed a PLMN phenotype more frequently than wild-type, C9orf72 and other rare genes mutated patients (57.1% vs. 10.8%, 4.2 and 0%, respectively, p value = 0.0001).
SQSTM1 mutated patients displayed a classic phenotype without cognitive deficits at the time of diagnosis, with a family history for dementia in 28.6% of patients (Table 3).
Cognitive deficits were significantly more frequent in C9orf72 than in wild-type patients (33.3% vs. 5%, p value = 0.0001). No cognitive deficits were found in patients carrying variants in other genes, with the exception of three mutated patients in TARDPB (c.883G > A p.Gly295Ser), TBK1 (c.225G > C p.Glu75Asp), and CHCHD10 (c.100C > T p.Pro34Ser), respectively.
Discussion
Genetics plays an important role in ALS and FTD, recognized as two diseases that form a broad neurodegenerative continuum. At least 10% and 40% of patients diagnosed with ALS and FTD are known to carry an autosomal dominant genetic mutation [35].
Given recent advances in gene therapy [36], it has become increasingly important to predict whether and which genes are involved in single ALS patients. However, ascertaining the genetic basis in patients diagnosed with ALS and/or FTD is a challenge, given the continuous discovery of new genes rarely associated with these diseases and the detection of patients with unresolved early-onset and positive family history [37, 38].
In this study, we presented the family inheritance features and the genetic landscape of an Italian cohort of adult onset ALS, providing the frequency of the four major ALS genes (C9orf72, SOD1, FUS, and TARDBP) and of others rare ones associated with ALS.
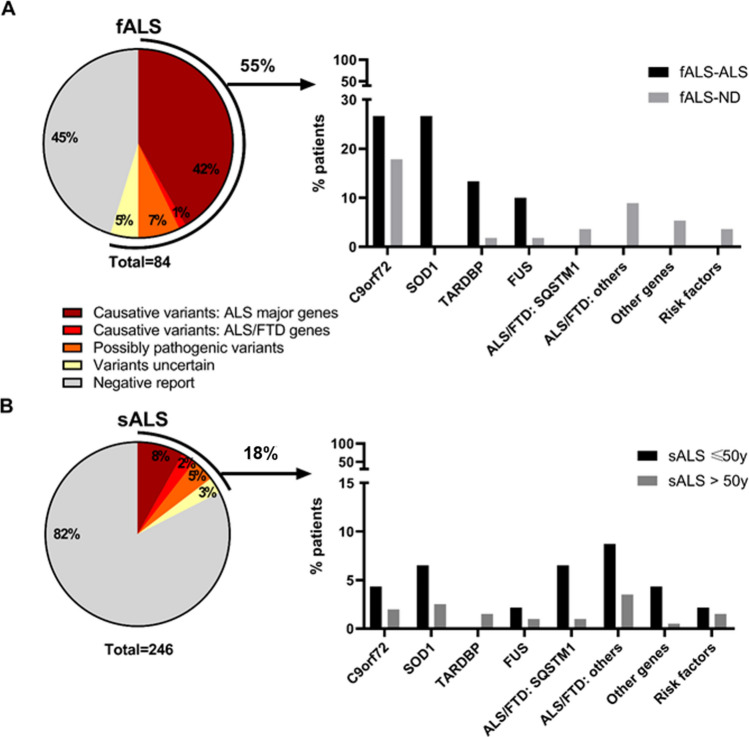
Adding up all the molecular analyses, we identified 55 patients with a causative o possibly causative variant in the four major ALS genes, 22 with a potentially pathogenic variant in rare ALS/FTD genes (novel likely pathogenic variants or previously reported variants in ALS patients), and 9 with variants defined as of uncertain significance (novel predicted to be likely pathogenic in genes linked to other neurodegenerative diseases or in ALS risk factor genes or heterozygous variants in ALS recessive genes), (Fig. 2a,b). Of these patients, five carried more than one variant, including one with a RE in the C9orf72 gene. In summary, 27.0% of the overall cohort carried an ALS-related variant, affecting 54.8% of fALS, and 17.5% of sALS patients (Fig. 2a,b).
Fig. 2.
Genetic variants distribution in our ALS population. Variants are classified as causative (ALS major genes and ALS/FTD genes), possibly pathogenic variants and variant of uncertain significant (VUS). The distribution of genetic variants is different between sALS and fALS patients, accounting for 17% of sALS and 55% of fALS patients. Genetic contribution was also different between fALS-ALS and fALS-ND, and between sALS, considering AAO. AAO age at onset; ALS amyotrophic lateral sclerosis; fALS familial ALS; fALS-ALS familial ALS with positive family history for ALS; fALS-ND familial ALS with positive family history for other neurodegenerative diseases; sALS sporadic
Despite differences in the NGS panel and pipeline used to classify variants, these results are consistent with those reported in other high-throughput sequencing studies performed in the ALS Italian population [10, 33].
A significant proportion (45%) of fALS patients remained without a genetic diagnosis, suggesting that other ALS genes may be involved and remain to be uncovered, possibly including, as for Alzheimer’s Diseases, rare family private pathogenic variants in risk factor genes [39–41].
For the correct application of genetic testing, counseling for each patient and the interpretation of results, family history, pedigree analysis, and risk assessment remain crucial [42]. The family history for ALS in our cohort, affecting 9.1% of the cohort, was comparable to that of previous studies [43, 44]. However, when the family history for other neurodegenerative diseases was also considered, as recommended [38], the rate increased to 25.5%.
The two types of family history followed two distinct trends associated with AAO (Fig. 1b), where the family history for ALS (fALS-ALS) was associated with a lower AAO, and those with fALS-ND (family history for other neurodegenerative diseases, i.e., dementia and parkinsonism) did not differ from sALS (Fig. S1), suggesting the involvement of distinct pathogenic mechanisms and/or genetic backgrounds in these two overlapping clinical syndromes [45–47].
As for the genetic landscape, the overall mutation frequency of the four major ALS genes was 41.7% in fALS and 8.1% in sALS, similar to those observed in a recent meta-analysis [48]. These explained 76.6% of fALS-ALS cases, but only 22.2% of fALS-ND patients. The RE in C9orf72 gene, the most frequent pathogenic mutation in our sample (Table 2) and in general in the ALS/FTD continuum in Europe [47], was associated with every phenotype of the ALS/FTD continuum and was evenly distributed in the two subgroups of fALS (Table 2).
In contrast, SOD1 mutations were identified only in fALS-ALS (26.7% of cases), and, similarly, mutations in FUS and TARDBP were preferentially linked to fALS-ALS (Table 2).
Concerning other genes (Table S1) the most frequently involved were those belonging to the ALS/FTD continuum, rarely causative of fALS forms [11]. Their variations were detected in 32 patients (15.5% of the analyzed samples). Among these, SQSTM1 proved to be the most frequently mutated gene, confirming previous finding in the Italian population [11] (n = 7, 3.4%, Table 3), followed by CHMP2B (n = 4, 1.9%, Table 3). SQSTM1 showed a behavior similar to that of RE C9orf72, sharing with the major ALS genes a young AAO of the affected patients, but with a rare family history for other neurodegenerative diseases (Tables 3, S6).
Other variants were found in CCNF, CHCHD10, DCTN1, FIG4, OPTN, SETX, TBK1, and UBQLN1, and each represented less than 1% of our cohort, as previously reported [10, 11]. Together, rare ALS/FTD genes accounted for 12% of our population.
Besides, eight other patients were carriers of a likely pathogenic variant in genes not related to the ALS/FTD continuum (APP, CSF1R, NOTCH3, ITM2B), but linked to other types of neurodegenerative dementia. According to the model that considers the neurodegenerative disease as a continuous variation in clinical/pathological features [49], these variants may be risk factors for ALS.
Recent studies highlighted the role of genetic risk factors in sALS patients, where heritability would represent about 21.0% [7]. Likewise, we found rare variants in the NEK1 gene, all in sALS, in 5.8% of the tested samples (3 patients out of 51) (Table 4).
Given the rarity of several mutations, we did not find any clinical features that allow us to define a specific screening pathway, as already highlighted in previous reports [42]. However, our data support the presence of at least two different pathogenetic components underlying the ALS phenotype based on the type of family history and suggest the implementation of a differentiated screening protocol.
As pointed out before, all ALS patients should be tested for RE in the C9Orf72 gene, given the extreme variability in familial occurrence and AAO, the relatively low cost of the test and the forthcoming of C9orf72-targeted therapeutic trials [47]. Furthermore, fALS-ALS patients should be screened for SOD1, FUS, and TARDBP, which in our cohort explain 50% of fALS-ALS cases, reaching 76.6% with C9orf72 testing (Fig. 2).
In contrast, patients with a family history for other neurodegenerative diseases were more likely to carry a mutation in other ALS/FTD genes (20% vs. 4.1% in fALS-ALS corresponding to only one patient, #ALS82, also carrying a SOD1 mutation) as an inheritable trait.
In our cohort, a pathogenic/probably pathogenic mutation in the four main ALS genes or the rare ALS/FTD genes was present in 8.1% and 10% of sALS, respectively (Fig. 2). fALS may resemble a sporadic disease due to incomplete penetration or incomplete family history. In the case of negative family history, some "red flags" such as early AAO (< 50 years), atypical rapid or slow progression of the disease, and the presence of dementia may suggest a familial form [50]. No other clinical features (ALS variant, dementia, etc.) have proven useful in suggesting the possible genetic origin.
Finally, since the diagnostic algorithms should be optimized according to ethnic origin, we confirm that in the Italian population SQSTM1 should be analyzed together with the four main genes [11].
In conclusion, despite decades of intensive research, ALS etiology remains unexplained, and the number of genes associated with disease risk and pathogenesis continues to grow. An NGS approach or exome/genome studies are not yet exhaustive and are suggested for research, hoping that they may help find a tailor-made treatment option for most ALS patients in the future. We suggest a protocol that could be useful in a clinical setting.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge all patients and their caregivers for supporting the research in ALS. They also thank the Italian Ministry of Research RFO, the “Fondazione del Monte”, the “Fondazione Gino Galletti”, and the “Fondazione Il Bene Onlus”. Finally, we are greatful to the BoReALS group, for the clinical care of patients and for support in collecting data: Ilaria Bartolomei, Franca Cinelli, Arianna Cherici, Vitantonio Di Stasi, Rosaria Plasmati, Francesca Pastorelli, Cecilia Celidea Quarta, David Milletti, Raffaella Nasca, Francesca Rizzi, Luca Valeriani, Francesca Anzolin, Elisabetta Fantoni, Patrizia Avoni, Vincenzo Donadio, Fiorito Alessia, Sabina Capellari, Silvia de Pasqua, Giovanni Rizzo, Veria Vacchiano, Enrico Fileccia, Luca Albini-Riccioli, Piero Parchi, Maria Pia Foschini, Annalisa Pession, Stella Battaglia, Sofia Asioli, Luca Vignatelli, Michelangelo Stanzani-Maserati, Anna Bartoletti-Stella, Carolina Colombo, Serena Maselli, Maria Pia Giannoccaro, Fabrizio Salvi, Rocco Liguori.
Author contribution
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript: A. Writing of the first draft, B. Review and Critique; Anna Bartoletti-Stella and Veria Vacchiano: 1A, 1B, 1C, 2A, 2B, and 3A. Rocco Liguori and Sabina Capellari: 1A, 1B, 1C, 2C, and 3B. Silvia De Pasqua, Giacomo Mengozzi, Dario De Biase, Ilaria Bartolomei, Patrizia Avoni, Giovanni Rizzo, Piero Parchi, Vincenzo Donadio, Adriano Chiò, Annalisa Pession, Federico Oppi, Fabrizio Salvi: 1C, 2C, 3B.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Data availability
The genetic data sets can be requested to the corresponding author. All clinical data are reported in the manuscript and in the supplementary data files.
Declaration
Conflicts of interest
The authors declare no conflict of interest.
Financial disclosure
R. Liguori reports personal fees from Biogen, Sanofi-Genzyme, Argon Healthcare s.r.l., Amicus Therapeutics s.r.l., and Alfasigma for Advisory Board consultancy and Lecture fees from Dynamicom Education, SIMG Service, Adnkronos salute unipersonale s.r.l., and DOC Congress s.r.l. outside the submitted work. The other authors do not have any disclosure to declare.
Ethics approval
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines and approved by the “Area Vasta Emilia Centro” ethics committee. Informed consent was given by study participants.
Footnotes
Anna Bartoletti-Stella and Veria Vacchiano contributed equally.
Rocco Liguori and Sabina Capellari share senior authorship.
References
- 1.Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3–4):153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattante S, Ciura S, Rouleau GA, Kabashi E. Defining the genetic connection linking amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (FTD) Trends Genet. 2015;31:263–273. doi: 10.1016/j.tig.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JP, Brown RH, Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahheydari H, Ragagnin A, Walker AK, et al. Protein quality control and the amyotrophic lateral sclerosis/frontotemporal dementia continuum. Front Mol Neurosci. 2017;10:119. doi: 10.3389/fnmol.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown C. Non-familial ALS: a tangled web. Nature. 2017;550(7676):S109–S111. doi: 10.1038/550S109a. [DOI] [PubMed] [Google Scholar]
- 7.Keller MF, Ferrucci L, Singleton AB, et al. Genome-wide analysis of the heritability of amyotrophic lateral sclerosis. JAMA Neurol. 2014;71(9):1123–1134. doi: 10.1001/jamaneurol.2014.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Chalabi A, Fang F, Hanby MF, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry. 2010;81(12):1324–1326. doi: 10.1136/jnnp.2010.207464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcia P, Couratier P, Blasco H, et al. Genetics of amyotrophic lateral sclerosis. Rev Neurol (Paris) 2017;173(5):254–262. doi: 10.1016/j.neurol.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Lamp M, Origone P, Geroldi A, et al. Twenty years of molecular analyses in amyotrophic lateral sclerosis: genetic landscape of Italian patients. Neurobiol Aging. 2018;66:179.e5–179.e16. doi: 10.1016/j.neurobiolaging.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Pensato V, Magri S, Bella ED, et al. Sorting rare ALS genetic variants by targeted re-sequencing panel in Italian patients: OPTN, VCP, and SQSTM1 variants account for 3% of rare genetic forms. J Clin Med. 2020;9(2):412. doi: 10.3390/jcm9020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita S, Ando Y. Genotype–phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener. 2015;4:13. doi: 10.1186/s40035-015-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannoccaro MP, Bartoletti-Stella A, Piras S, et al. Multiple variants in families with amyotrophic lateral sclerosis and frontotemporal dementia related to C9orf72 repeat expansion: further observations on their oligogenic nature. J Neurol. 2017;264(7):1426–1433. doi: 10.1007/s00415-017-8540-x. [DOI] [PubMed] [Google Scholar]
- 14.Marangi G, Traynor BJ. Genetic causes of amyotrophic lateral sclerosis: new genetic analysis methodologies entailing new opportunities and challenges. Brain Res. 2015;1607:75–93. doi: 10.1016/j.brainres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10(11):661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- 17.Chiò A, Moglia C, Canosa A, et al. ALS phenotype is influenced by age, sex, and genetics: a population-based study. Neurology. 2020;94(8):e802–e810. doi: 10.1212/WNL.0000000000008869. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 19.Gallassi R, Lenzi P, Stracciari A, et al. Neuropsychological assessment of mental deterioration: purpose of a brief battery and a probabilistic definition of “normality” and “non-normality”. Acta Psychiatr Scand. 1986;74:62–67. doi: 10.1111/j.1600-0447.1986.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 20.Boylan K. Familial amyotrophic lateral sclerosis. NeurolClin. 2015;33(4):807–830. doi: 10.1016/j.ncl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visani M, de Biase D, Bartolomei I, et al. A novel T137A SOD1 mutation in an Italian family with two subjects affected by amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12(5):385–388. doi: 10.3109/17482968.2011.582648. [DOI] [PubMed] [Google Scholar]
- 22.Bartoletti-Stella A, Baiardi S, Stanzani-Maserati M, et al. Identification of rare genetic variants in Italian patients with dementia by targeted gene sequencing. Neurobiol Aging. 2018;66:180.e23–180.e31. doi: 10.1016/j.neurobiolaging.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner MR, Barnwell J, Al-Chalabi A, Eisen A. Young-onset amyotrophic lateral sclerosis: historical and other observations. Brain. 2012;135(Pt 9):2883–2891. doi: 10.1093/brain/aws144. [DOI] [PubMed] [Google Scholar]
- 26.Espay AJ, Litvan I. Extrapyramidal syndrome and frontotemporal dementia: the clinical overlap. J Mol Neurosci. 2011;45(3):343–349. doi: 10.1007/s12031-011-9632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacoangeli A, Al Khleifat A, Jones AR, et al. C9orf72 intermediate expansions of 24–30 repeats are associated with ALS. Acta Neuropathol Commun. 2019;7(1):115. doi: 10.1186/s40478-019-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gellera C, Castellotti B, Riggio MC, et al. Superoxide dismutase gene mutations in Italian patients with familial and sporadic amyotrophic lateral sclerosis: identification of three novel missense mutations. Neuromuscul Disord. 2001;11(4):404–410. doi: 10.1016/S0960-8966(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 29.Gamez J, Corbera-Bellalta M, Nogales G, et al. Mutational analysis of the Cu/Zn superoxide dismutase gene in a Catalan ALS population: should all sporadic ALS cases also be screened for SOD1? J Neurol Sci. 2006;247(1):21–28. doi: 10.1016/j.jns.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Giannini F, Battistini S, Mancuso M, et al. D90A-SOD1 mutation in ALS: the first report of heterozygous Italian patients and unusual findings. Amyotroph Lateral Scler. 2010;11(1–2):216–219. doi: 10.3109/17482960902721642. [DOI] [PubMed] [Google Scholar]
- 31.Hosler BA, Nicholson GA, Sapp PC, et al. Three novel mutations and two variants in the gene for Cu/Zn superoxide dismutase in familial amyotrophic lateral sclerosis. Neuromuscul Disord. 1996;6(5):361–366. doi: 10.1016/0960-8966(96)00353-7. [DOI] [PubMed] [Google Scholar]
- 32.Dong SQ, Liu XN, Yang WB, Zhou YN, Wang JC, Chen XJ (2020) An exon 5 mutation (c.425G>C, p.Gly141Ala) in the SOD1 gene in a Chinese family associated with incomplete penetrance [published online ahead of print, 2020 Mar 15]. Amyotroph Lateral Scler Frontotemporal Degener1–4 [DOI] [PubMed]
- 33.Lattante S, Conte A, Zollino M, et al. Contribution of major amyotrophic lateral sclerosis genes to the etiology of sporadic disease. Neurology. 2012;79(1):66–72. doi: 10.1212/WNL.0b013e31825dceca. [DOI] [PubMed] [Google Scholar]
- 34.Münch C, Rolfs A, Meyer T. Heterozygous S44L missense change of the spastin gene in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9(4):251–253. doi: 10.1080/17482960801900172. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari R, Kapogiannis D, Huey ED, Momeni P. FTD and ALS: a tale of two diseases. Curr Alzheimer Res. 2011;8(3):273–294. doi: 10.2174/156720511795563700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappella M, Ciotti C, Cohen-Tannoudji M, Biferi MG. Gene therapy for ALS-A perspective. Int J Mol Sci. 2019;20(18):4388. doi: 10.3390/ijms20184388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson SB, Downie JM, Tsetsou S, et al. The evolving genetic risk for sporadic ALS. Neurology. 2017;89(3):226–233. doi: 10.1212/WNL.0000000000004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vajda A, McLaughlin RL, Heverin M, et al. Genetic testing in ALS: a survey of current practices. Neurology. 2017;88(10):991–999. doi: 10.1212/WNL.0000000000003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer's disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138(2):201–220. doi: 10.1007/s00401-019-01994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen HP, Van Broeckhoven C, van der Zee J. ALS genes in the genomic era and their implications for FTD. Trends Genet. 2018;34(6):404–423. doi: 10.1016/j.tig.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrone B, Conforti FL (2020) Common mutations of interest in the diagnosis of amyotrophic lateral sclerosis: how common are common mutations in ALS genes? [published online ahead of print, 2020 Jun 16]. Expert Rev Mol Diagn 1–12 [DOI] [PubMed]
- 43.Logroscino G, Traynor BJ, Hardiman O, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385–390. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masrori P, Van Damme P (2020) Amyotrophic lateral sclerosis: a clinical review [published online ahead of print, 2020 Jun 11]. Eur J Neurol. 10.1111/ene.14393 [DOI] [PMC free article] [PubMed]
- 45.Tan RH, Ke YD, Ittner LM, Halliday GM. ALS/FTLD: experimental models and reality. Acta Neuropathol. 2017;133(2):177–196. doi: 10.1007/s00401-016-1666-6. [DOI] [PubMed] [Google Scholar]
- 46.Karch CM, Wen N, Fan CC, et al. Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol. 2018;75(7):860–875. doi: 10.1001/jamaneurol.2018.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roggenbuck J, Quick A, Kolb SJ. Genetic testing and genetic counseling for amyotrophic lateral sclerosis: an update for clinicians. Genet Med. 2017;19(3):267–274. doi: 10.1038/gim.2016.107. [DOI] [PubMed] [Google Scholar]
- 48.Zou ZY, Zhou ZR, Che CH, Liu CY, He RL, Huang HP. Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2017;88(7):540–549. doi: 10.1136/jnnp-2016-315018. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong RA. On the 'classification' of neurodegenerative disorders: discrete entities, overlap or continuum? Folia Neuropathol. 2012;50(3):201–208. doi: 10.5114/fn.2012.30521. [DOI] [PubMed] [Google Scholar]
- 50.Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim Biophys Acta. 2015;1852(4):679–684. doi: 10.1016/j.bbadis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Yilmaz R, Müller K, Brenner D, et al. SQSTM1/p62 variants in 486 patients with familial ALS from Germany and Sweden. Neurobiol Aging. 2020;87:139.e9–139.e15. doi: 10.1016/j.neurobiolaging.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Giannoccaro MP, Bartoletti-Stella A, Piras S, et al. The first historically reported Italian family with FTD/ALS teaches a lesson on C9orf72 RE: clinical heterogeneity and oligogenic inheritance. J Alzheimers Dis. 2018;62(2):687–697. doi: 10.3233/JAD-170913. [DOI] [PubMed] [Google Scholar]
- 53.D'Amore A, Tessa A, Casali C, et al. Next generation molecular diagnosis of hereditary spastic paraplegias: an Italian cross-sectional study. Front Neurol. 2018;9:981. doi: 10.3389/fneur.2018.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genetic data sets can be requested to the corresponding author. All clinical data are reported in the manuscript and in the supplementary data files.