Abstract
A 2 year old boy presented with a large cystic and solid chest mass arising from the lung, radiographically consistent with pleuropulmonary blastoma (PPB). He underwent right lower lobectomy with resection of a well-circumscribed, mixed solid and cystic mass. The solid areas were composed of cords and nests of tumor cells in the myxoid stroma and retiform foci whose pathologic and immunophenotypic findings were consistent with a sex cord-stromal tumor with features of a Sertoli-Leydig cell tumor. Tumor testing showed a pathogenic variant in the DICER1 RNase IIIb hotspot domain. Family history was suggestive of DICER1 germline pathogenic DICER1 variation in absence of a detectable germline variant. He received 12 cycles of chemotherapy with ifosfamide, vincristine, doxorubicin and dactinomycin (IVADo) and surgery with complete response. One year after completion of chemotherapy, imaging studies showed concern for recurrence confirmed by thorascopic biopsy of a pleural-based mass. He is currently receiving cisplatin-based chemotherapy with reduction in tumor size. Review of the literature showed no similar cases, however, review of our pathology files revealed a single similar case of anterior mediastinal Sertoli cell tumor in a 3 year old girl.
Keywords: Sertoli-Leydig cell tumor, thoracic tumor, pleuropulmonary blastoma, DICER1
Introduction:
Since the identification of a heterozygous germline pathogenic variant in DICER1 in children with PPB, a spectrum of distinctive extrapulmonary neoplasms has emerged as related to germline or somatic DICER1 pathogenic variation.1,2. One of these is ovarian Sertoli-Leydig cell tumor (SLCT)3. The current case illustrates the modeled plasticity of the DICER1-associated neoplasms irrespective of the primary site, as in the case of a primary SLCT of the lung.
Brief Reports:
Case 1.
A 2 year old boy presented with respiratory distress and was found to have a large mixed cystic and solid thoracic mass with mediastinal shift (Fig. 1A), initially thought to represent Type II PPB. A right lower lobe resection revealed a neoplasm centered in the lung without involvement beyond the bronchial margins (Fig. 1B), but with microscopic extension into the mediastinum adjacent to the thymus. Staging studies showed no residual thoracic disease, no evidence for metastatic disease and absence of a testicular mass. Family history showed a paternal uncle with history of cystic nephroma resected at age 2 and a paternal aunt with a history of follicular variant of papillary thyroid carcinoma diagnosed at age 8.
Figure 1.
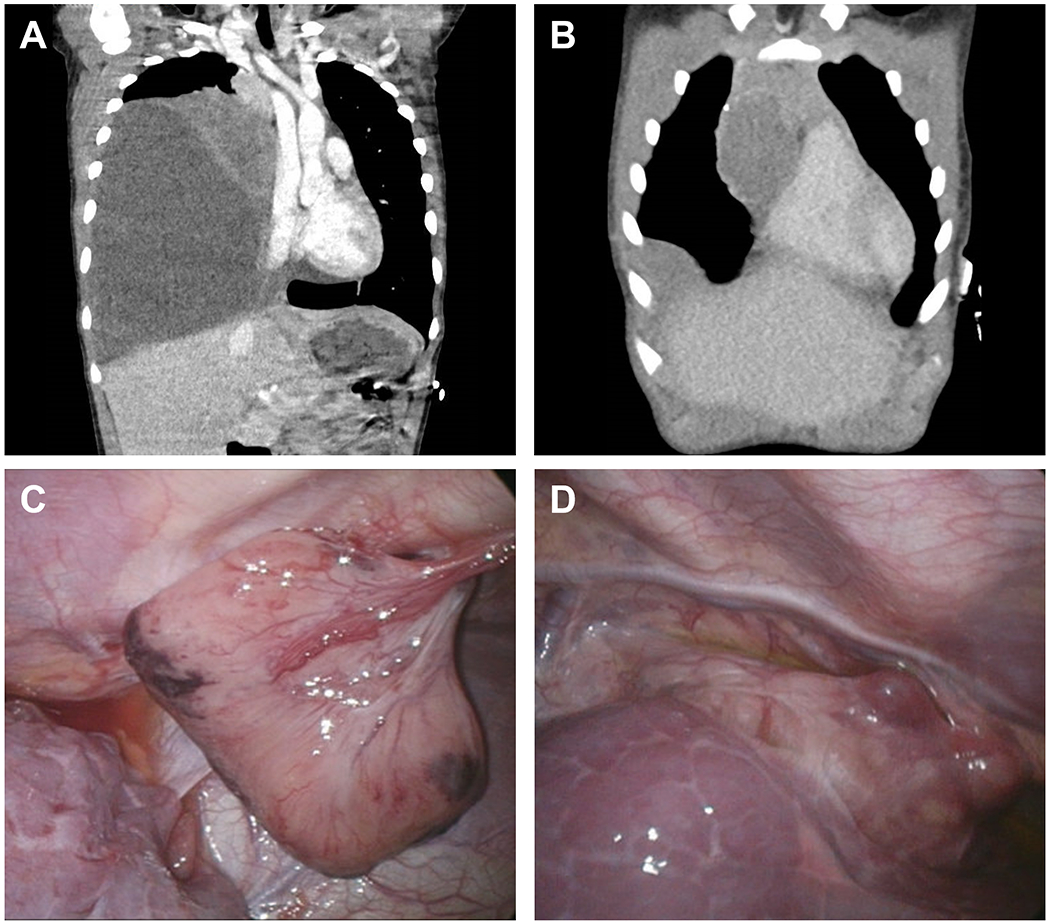
A. Computed tomography (CT) scan at initial presentation demonstrating a mixed cystic and solid mass. B. Surveillance CT scan performed one year following diagnosis, demonstrating recurrent mediastinal mass and pleural-based lesion. C. Intraoperative photo obtained during thorascopic biopsy of recurrent anterior chest wall pedunculated mass. D. Intraoperative photo obtained during thorascopic excision of anterior mediastinal pleural based mass.
Though the tumor was not a PPB, its alternative features nonetheless prompted DICER1 testing which demonstrated a somatic DICER1 variant in the RNase IIIb (hotspot) region in absence of detectable germline variant. Adjuvant chemotherapy was initiated with 12 cycles of ifosfamide, vincristine, dactinomycin and doxorubicin (IVADo). He tolerated therapy well but developed selective mutism of unclear etiology 3 months after completing therapy. Imaging at end of therapy showed no evidence for residual disease, but surveillance scans one year later revealed anterior mediastinal and pleural-based masses. Thorascopic biopsy of the pleural-based lesion showed pathologic and immunohistochemical findings similar to the primary tumor (Figs. 1C and 1D). He is currently receiving chemotherapy with cisplatin, etoposide and bleomycin with reduction in tumor size.
Case 2.
We identified an additional similar case arising in the anterior mediastinum/thymus of a 3 year old girl. Though the microscopic and immunophenotypic features were similar, molecular studies were not available.
Pathology:
Case 1.
The right lower lobe was replaced by a 16.5 x 13 x 6 cm, solid and cystic, brownish-tan and focally hemorrhagic mass which abutted the pleura, but did not invade into or through it, but the tumor extended into the adjacent soft tissues without involvement of the thymus. The septa in the cystic areas were composed of primitive small mesenchymal cells with patchy desmin and myogenin immunopositivity. In the solid foci, two patterns were noted, one of which was characterized by discrete nests of Sertoli cells with the interspersed eosinophilic cells and a surrounding hypocellular myxoid stroma and more compactly cellular areas of small tubular and papillary retiform profiles (Figs. 2A and 2B). These solid foci were diffusely immunoreactive for inhibin and WT-1 (Figs. 2C and 2D). Additionally the tumor was positive for calretinin, and SF-1 vimentin and CAM5.2 (not shown). The tumor was non-reactive for estrogen and progesterone receptors and B-catenin.
Figure 2.
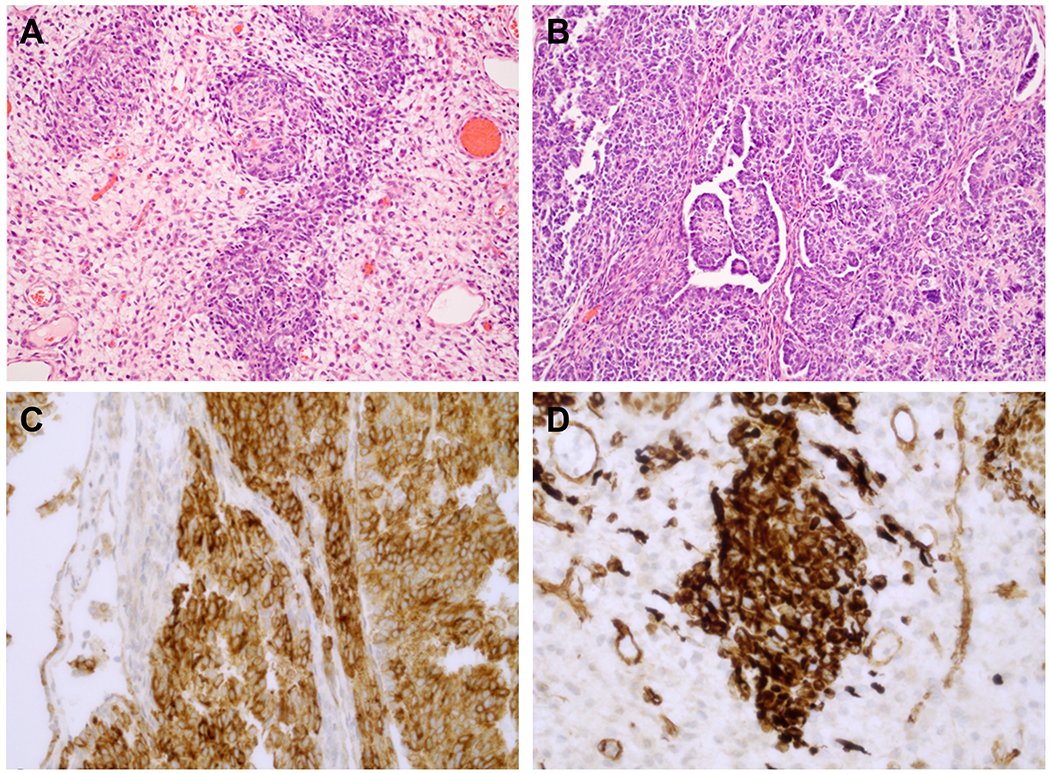
A. Small cellular nests of tumor surrounded by a pale myxoid stroma. Within the nests, collections of small cells are accompanied by larger eosinophilic cells with features of Leydig cells. B. More cellular areas were composed of small tubules and cords of basophilic cells with features of Sertoli cells and papillary-retiform features. C. Diffuse, intense inhibin immunopositivity is present. D. WT1 nuclear positivity highlighted the nested foci.
Molecular studies showed an activating mutation in the RNA IIIb (hotspot) domain (D1813) in the absence of a detectable germline pathogenic variant, duplication or deletion. Germline DICER1 array comparative genomic hybridization (aCGH) is pending. There were no mutations in SMARCA4 and BRG-1 expression was preserved by IHC. Breakapart FISH for 18q11.3 rearrangement was negative.
Case 2 (3 year old girl identified from case files):
Sections from the tumor arising in the mediastinum demonstrated a sharply demarked neoplasm arising from the surrounding thymus composed of cords, trabecula and tubules of moderately sized cells with mildly pleomorphic, overlapping nuclei and pale, basophilic cytoplasm. There were numerous mitotic figures and karyorrhectic figures present as well as small glandular or tubular profiles with a retiform appearance. Focally, a pale, pink collagenous stroma was noted, reminiscent of the type of stroma associated with a sex cord-mesenchymal neoplasm. Immunophenotype was consist with sex cord neoplasm with staining for cytokeratin, inhibin and WT1.
Discussion:
The two cases presented in this report document the extremely rare phenomenon of tumors arising in an ectopic location well beyond the expected primary site and in the absence of a primary gonadal neoplasm.
The morphologic spectrum of sex cord stromal tumors of the ovary is known to occur in the testis, but these tumors are considerably less common than their ovarian counterparts5,6. Juvenile granulosa cell tumor (JGCT) is the most common sex cord stromal tumor of the testis with its earliest clinical presentation in infancy7. In contrast, SLCT in this site is considerably less well documented in the literature.
In a recent study from the International PPB/DICER1 and Ovarian Testicular Stromal Tumor Registries, testing of SLCTs revealed DICER1 pathogenic variation in virtually all cases, with loss of function and hotspot mutations9. Approximately half of SLCTs of the ovary arose in an individual with a germline pathogenic variation or mosaicism. A recent report also documents the potential relationship between testicular sex cord stromal tumor and DICER1 pathogenic variation10,11.
To the best of our knowledge, an ectopic, non-metastatic SLCT or another type of sex cord-stromal tumor presenting in the lung and/or mediastinum has not been reported to date. There are two cases of extraovarian primary mesenteric and one case of an extratesticular SLCT in the pelvis, but no examples beyond the abdominal cavity12–14.
As one searches for a non-metastatic explanation for the sex cord stromal tumors in these patients, development from ectopic genital ridge epithelium-mesenchyme becomes a consideration similar to the migratory primordial germ cells and the well-established extragonadal presentation of germ cell neoplasms. As sex cord-stromal tumors are not typically found within the thoracic cavity and PPBs do not typically contain Sertoli or Leydig cells, we considered the possibility that this tumor represents a germ cell tumor containing sex cord-stromal tumors with malignant transformation, however, there were no apparent germ cell elements. Additionally, the DICER1 alteration suggests a primary sex cord-stromal tumor. Based on these features, it seems more likely that site of the pathogenic variant in DICER1 offers the best explanation for a group of neoplasms which arise in a variety of organs and locations from the lung, as in the case of the PPB, to the central nervous system, kidney, female genital tract including the ovarian SLCT and pelvic peritoneum2,15,16. In several of these primary sites, the neoplasms have overlapping pathologic features as though there is a morphologic blueprint for these tumors. We would argue that one of these motifs is a tumor in the ovary that is recognized as SLCT17. Just as we encounter the PPB-like pattern in the kidney as pediatric cystic neoplasm which may progress to anaplastic sarcoma18,19, so a DICER1 pathogenic variation regardless of its germline or somatic origin can evolve into a neoplasm whose pathologic and immunophenotypic features are those of SLCT as in our case. This serves as a potential biologic explanation for a known DICER1 neoplasm arising in an aberrant site rather than a more conventional, but less plausible embryologic scenario. Like Type II PPB, the cystic foci of our case resembled Type I PPB with patchy rhabdomyoblastic differentiation, but rather than progressing to the complex, primitive multipatterned sarcoma of the solid component of PPB, the tumor pursued the histogenesis of a SLCT and locally recurred as the latter.
In this report, we present two cases of sex cord-stromal tumors arising in the anterior mediastinum, one SLCT and the other a Sertoli cell tumor. In the first case, DICER1 pathogenic variation was detected. This tumor had the solid and cystic features of type II PPB, but solid pattern was that of a moderate or intermediately differentiated SLCT. The optimal treatment in these clinical scenarios remains indeterminate; additional studies are required. These manifestations also highlight the need for careful ascertainment of family history and DICER1 testing for any sex cord-stromal tumor regardless of primary site20
Acknowledgments:
The authors wish to thank the many treating physicians, genetic counselors, patients and families who collaboratively support the International PPB/DICER1 Registry and the Pine Tree Apple Classic Fund whose volunteers, tennis players and donors have provided more than 30 years of continuous support for PPB and DICER1 research. Specifically, this work was supported by the Pine Tree Apple Tennis Classic Fund. The International PPB/DICER1 Registry is also supported by the Children’s Minnesota Foundation and Rein in Sarcoma. KAS, DAH and PM also receive support from NIH/NCI 1R37CA244940.
Abbreviations:
- PPB
pleuropulmonary blastoma
- IVADo
Ifosfamide, vincristine, doxorubicin and dactinomycin
- SLCT
Sertoli-Leydig cell tumor
- aCGH
array comparative genomic hybridization
- JGCT
Juvenile granulosa cell tumor
- CT
Computed tomography
Footnotes
Conflict of Interest: DAH is founder/investor in ResourcePath LLC. The other authors have no conflicts of interest to disclose.
References
- 1.Hill DA, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science, 2009; 325(5943):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz KAP, et al. DICER1 tumor predisposition. In: GeneReviews®. University of Washington, Seattle; 1993-2021. [PubMed] [Google Scholar]
- 3.Schultz KAP, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: a report from the International Pleuropulmonary Blastoma Registry. Gyneco Oncol, 2011; 122(2):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young RH Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol, 2005; 18Suppl 2:S81–98. [DOI] [PubMed] [Google Scholar]
- 5.Fresneau B, et al. Sex-cord stromal tumors in children and teenagers: results of the TGM-95 study. Pediatr Blood Cancer, 2015; 62(12):2114–2119. [DOI] [PubMed] [Google Scholar]
- 6.Schneider DT, et al. Consensus recommendations from the EXPeRT/PARTNER groups for the diagnosis and therapy of sex cord stromal tumors in children and adolescents. Pediatr Blood Cancer, 2021; March24;e29017. doi:10.1007. [DOI] [PubMed] [Google Scholar]
- 7.Roth LM, Lyu B, Cheng L Perspectives on testicular sex cord-stromal tumors and those composed of both germ cells and sex cord-stromal derivatives with a comparison to corresponding ovarian neoplasms. Hum Pathol, 2017; 65:1–14. [DOI] [PubMed] [Google Scholar]
- 8.Heravi-Moussavi A, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med, 2012. 366(3): p. 234–42. [DOI] [PubMed] [Google Scholar]
- 9.Schultz KAP, et al. , DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: Clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol Oncol, 2017. 147(3):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golmard L, et al. Testicular Sertoli cell tumor and potentially testicular Leydig cell tumor are features of DICER1 syndrome. J Med Genet, 2021, March29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlon N, et al. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mot Pathol, 2015; 28(12):1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osakabe M, et al. , Mesenteric extraovarian Sertoli-Leydig cell tumor without DICER1 hotspot mutation: a case report. Diagn Pathol, 2019; 14(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trabelsi A, et al. , Primary mesenteric Sertoli-Leydig cell tumor: a case report and review of the literature. J Oncol, 2008:619637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer R, et al. , Extratesticular gonadal stomal tumor in the pelvis. A case report with immunoperoxidase findings. Cancer, 1980. 45(5):985–90. [DOI] [PubMed] [Google Scholar]
- 15.Brenneman M, et al. , Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: a unique variant of the two-hit tumor suppression model. F1000Res, 2015; 4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehner LP, et al. Pleuropulmonary blastoma: more than a lung neoplasm of childhood. Mo Med, 2019; 116(3):206–210. [PMC free article] [PubMed] [Google Scholar]
- 17.Young RH, Scully RE Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol, 1985; 9(8):543–569. [DOI] [PubMed] [Google Scholar]
- 18.Doros LA, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol, 2014; 27(9):1267–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MK, et al. Tumor progression in DICER1-mutated cystic nephroma-witnessing the genesis of anaplastic sarcoma of the kidney. Hum Pathol, 2016; 53:114–120. [DOI] [PubMed] [Google Scholar]
- 20.Schultz KAP, et al. , DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res, 2018. 24(10):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


