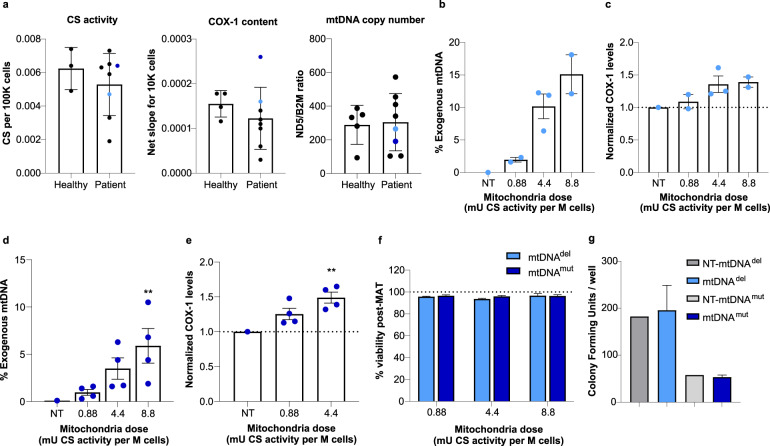
Fig. 2. Mitochondrial augmentation of patient-derived HSPCs is a dose-dependent process and does not impair cell viability or functionality.
a Mitochondrial activity or content of healthy and patient-derived HSPCs is variable, as quantitated by CS activity, COX-1 content, and mtDNA copy number. (p = 0.51, t(8) = 0.6837, n = 3 (healthy CD34+) and n = 8 (patient CD34+) for CS; p = 0.3995, t(10) = 0.8799 for COX-1 in unpaired t-test analysis, n = 4 (healthy CD34+) and n = 8 (patients’ CD34+). mtDNA copy number: p = 0.855, t(11) = 0.1864, n = 5 (healthy CD34+), n = 8 (patients’ CD34+) in unpaired t-test analysis. Light and dark blue dots represent the values for patient-derived samples used in experiments (b, c, light blue), and (d, e, dark blue). b, c mtDNAdel (Pearson Syndrome) patient CD34+ cells were augmented for 21 h with increasing doses of placenta-derived mitochondria. b Exogenous DNA analysis (n = 2 for 0.88 and 8.8 mU CS activity doses, n = 3 for 4.4 mU CS activity dose). c COX-1 analysis (single patient cells; n = 2 for 0.88 and 8.8 mU doses, n = 3 for 4.4 mU CS dose). d, e mtDNAmut (Leigh Syndrome) patient CD34+ cells were augmented for 21 h with increasing doses of placenta-derived mitochondria batches. d Exogenous mtDNA content following mitochondrial augmentation were determined using NGS (single patient’s cells with n = 4 for each mitochondrial dose tested, p < 0.0001 for the difference between four experimental groups, Friedman test (F-stat = 12.00), **p = 0.003 for 8.8 mU CS activity dose, analyzed by Dunn’s pot hoc multiple comparison. e COX-1 levels were determined using ELISA (single patient’s cells with n = 4 for each mitochondrial dose tested, p = 0.0046 for the difference between four experimental groups, Friedman test (F-stat = 8.00) followed by Dunn’s post hoc test: **p = 0.0094 for 4.4 mU CS activity dose). f CD34+ cells from mtDNAdel (Pearson Syndrome) patient or mtDNAmut (Leigh Syndrome) patient were augmented with increasing doses of placenta-derived mitochondria. Viability was assessed 21 h post augmentation using nucleocounter (mtDNAdel (single cell donor): 0.88, n = 2; 4.4, n = 3; 8.8, n = 2. MtDNAmut (single cell donor): n = 4 for all doses). g CD34+ cells from mtDNAdel patient or mtDNAmut patient were augmented with placenta-derived mitochondria. Ability to form colonies was tested 14 days (±2) post augmentation (mtDNAdel single cell donor; n = 3 placental mitochondria batches, p = 0.75, Wilcoxon matched-pairs signed rank test), mtDNAmut (single cell donor): n = 4 placental mitochondria batches, ns vs. non-augmented cells, p = 0.625, Wilcoxon matched-pairs signed rank test). CS citrate synthase, HSPCs hematopoietic stem and progenitor cells, mtDNA mitochondrial DNA, NGS next-generation sequencing, NT non-treated.