Abstract
The study was conducted to evaluate the effects of graded levels of Eimeria maxima challenge on endogenous loss, apparent ileal digestibility (AID), and standard ileal digestibility (SID) of amino acids. A total of 768 fourteen-day-old male Cobb 500 broiler chickens were randomly allocated into 64 battery cages. Apart from the regular corn-soybean based diet, the nitrogen-free diet (NFD) was formulated to determine the endogenous loss of amino acids. One-half of the birds (32 cages) were fed the NFD, and another half fed the regular diet from d 14 to 20. Both groups were further assigned to 4 treatments (nonchallenged control or three levels of challenge doses) with 8 replicate cages. The challenge doses were: the low challenge dose (Low) with E. maxima 12,500 oocysts, the medium challenge dose (Medium) with 25,000 E. maxima oocysts, and the high challenge dose (High) with 50,000 E. maxima oocysts. At 6 d postinfection, ileal digesta samples were collected and the intestinal lesion score were recorded. The results indicated a significant linear increase of endogenous amino acid flow in response to the graded E. maxima challenge. Moreover, the AID and SID of amino acids were linearly reduced due to the increasing challenge dose. The study demonstrated that NFD significantly reduced lesion scores, underestimating the true endogenous losses of birds fed regular diets. Even though the endogenous loss of amino acids was underestimated, they were linearly increased in response to the graded E. maxima challenge. In conclusion, the higher Eimeria dose birds were challenged with, the more endogenous amino acids were released into the intestine and the lower dietary nutrients were digested and absorbed by broiler chickens.
Key words: coccidiosis, Eimeria maxima, endogenous loss, standard ileal digestibility, apparent ileal digestibility
INTRODUCTION
Poultry nutritionists depend on precision and accuracy of feed ingredient nutrient content and its digestibility to formulate balanced diets for chickens. Formulating feed based on digestible amino acids improves feed efficacy and minimize nutrient excretion to the environment. The use of apparent ileal digestibility (AID) of energy and amino acids is widely accepted in diet formulation (Ravindran, 2021). Apart from providing information for formulating diets, AID is also a useful parameter to investigate intestinal health of animals under pathogen infection.
However, the AID underestimates the true nutrient digestibility because it does not account for the endogenous loss of nutrients. The endogenous amino acids primarily originate from sloughed epithelial cells, mucin, and digestive enzyme secretions (Nyachoti et al., 1997). They are mainly composed of 2 proportions, the basal and diet-specific endogenous losses. The basal endogenous flows are stable and only regulated by feed dry matter intake, whereas the specific endogenous losses can be induced by feed ingredients such as fiber and antinutritional factors (Stein et al., 2007). Several methodologies have been tested successfully to measure the basal endogenous loss of amino acids, including using nitrogen-free diets (NFD), highly-digestible protein diets, and the regression method (Furuya and Kaji, 1989; Siriwan et al., 1993). Several review articles have concluded the advantages and disadvantages of each approach (Stein et al., 2007; Adedokun et al., 2011; Ravindran, 2021). Among these methodologies, the NFD is the most widely used because it is relatively simple to conduct and has presented the most consistent results (Adedokun et al., 2007).
Standard ileal digestibility (SID) was obtained by correcting apparent ileal digestibility for basal endogenous amino acid flow as described by Stein et al. (2007). It should be noted that even the SID does not include the diet-specific endogenous losses in the calculation. In the authors’ knowledge there is no specific procedure available to directly measure specific amino acid losses. Although there are some methods to estimate “total” endogenous amino acid losses with the homoarginine method or isotope-labeled markers in advance, then subtracting the basal endogenous losses from the total amino acid losses (Stein et al., 2007).
Recently, pathogen-induced endogenous losses have been reported in a previous study (Adedokun et al., 2012). The author demonstrated that the endogenous loss of amino acid was increased by coccidiosis because of the sloughed epithelial cells and mucin secretion. Coccidiosis is a ubiquitous enteric disease in poultry infected by Eimeria species (Allen and Fetterer, 2002). It has caused profound economic loss due to bird mortality, poor growth performance, and treatments to control coccidiosis. The decrease of feed efficiency in birds infected with Eimeria can be attributed to intestinal destruction and suppressed nutrient absorption. Moreover, increasing the severity of infection linearly decreased the AID of energy, amino acids, and minerals (Rochell et al., 2016; Teng et al., 2020a). Although the AID is defined as the net disappearance of nutrients from the digestive tract proximal to the distal intestine (Stein et al., 2007), both diet and pathogen-induced endogenous losses are mixed in the ileal contents with undigested nutrients; therefore, reduced AID of birds infected with coccidiosis was attributed to not only the undigested diet but also the endogenous losses.
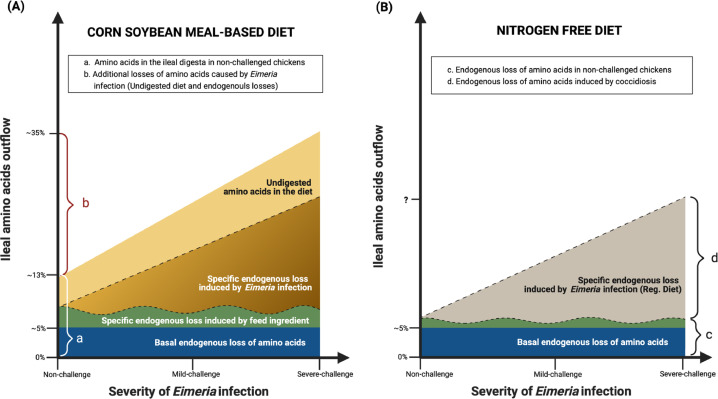
We hypothesized that the increasing infection severity of coccidiosis would linearly induce pathogen-specific endogenous losses (Figure 1A, Brown area) and increase undigested nutrients remaining in the distal ileal digesta (Figure 1A, Yellow area). Thus, the objective of the study was to evaluate the effects of graded coccidiosis infection on endogenous loss, AID, and SID of amino acids. Furthermore, the current study further investigated if the NFD method can determine the pathogen-induced endogenous losses in response to the graded infection severity (Figure 1B). With the correction of basal and pathogen-induced endogenous losses by the NFD method, the SID of amino acids would be further calculated and it is expected that applying the SID in feed formulation would improve feed efficiency under various magnitudes of Eimeria infection.
Figure 1.
The hypothesis of increased amino acid in the ileal digesta in response to the graded E. maxima infection severity. (A) Birds fed the corn-soybean meal diet; (B), birds fed the nitrogen-free diet.
MATERIAL AND METHODS
Experimental Design
The present study was approved by the Institutional Animal Care and Use Committee and conducted at the Poultry Research Center, University of Georgia, Athens, GA. A total of 768 fourteen-day-old male broiler chickens (Cobb 500) were used in the study. The experimental chickens were randomly allotted to 64 battery cages. The 64 cages were further divided into 2 groups (corn/soybean meal or nitrogen free diets). In the first group, 32 cages were randomly allocated to 4 treatments with 8 replicates and 12 birds in each cage. There were 4 treatments, including a nonchallenged control group (Control), a low E. maxima-challenged group (Low), a medium E. maxima-challenged group (Medium), and a high E. maxima-challenged group (High). At the beginning of the study, chickens in the Low, Medium, and High challenge groups were challenged with 12,500, 25,000, or 50,000 sporulated oocysts of E. maxima, respectively, and all of the experimental birds were fed ad libitum the corn-soybean meal diet in the first group.
On the other hand, the second group of the study was designed for measuring the endogenous loss of amino acids. Similar to the experimental arrangement in the first group, 32 cages were allotted to 4 treatments with 8 replicates. The birds were gavaged with the same graded levels of E. maxima oocysts in the challenged treatments as described above. Chickens in the second group were fed the NFD diet for 6 d (d 14–20) to measure the basal flow of amino acids in the intestine. Formulation of NFD was according to a balanced ions composition described by Adedokun et al. (2011) (Table 1). Moreover, the corn-soybean meal diet and the NFD were both formulated with 0.3% titanium dioxide as an indigestible indicator for determining AID and standard digestibility of amino acids. Chickens from both groups were raised in a house where the environmental program followed the Cobb Broiler Management Guide (Cobb, 2018).
Table 1.
Feed formulation of regular diet and nitrogen-free diet (%).
| Ingredient | Regular diet | Nitrogen-free diet |
|---|---|---|
| Corn | 59.41 | 0.00 |
| Soybean meal | 33.64 | 0.00 |
| Soybean oil | 1.88 | 5.00 |
| Corn starch | 0.00 | 20.05 |
| Dextrose | 0.00 | 64.00 |
| Fiber | 0.00 | 5.00 |
| Dicalcium phosphate | 1.59 | 0.00 |
| Limestone | 1.17 | 1.30 |
| Sodium chloride | 0.35 | 0.00 |
| DL-Methionine | 0.30 | 0.00 |
| L-Lysine HCl | 0.24 | 0.00 |
| Threonine | 0.09 | 0.00 |
| Sodium bicarbonate | 0.00 | 0.75 |
| Potassium chloride | 0.00 | 0.29 |
| Potassium carbonate | 0.00 | 0.26 |
| Magnesium oxide | 0.00 | 0.20 |
| Choline chloride | 0.00 | 0.25 |
| Monocalcium phosphate | 0.00 | 1.90 |
| Vitamin premix1 | 0.25 | 0.25 |
| Mineral premix2 | 0.08 | 0.08 |
| Titanium dioxide | 0.30 | 0.30 |
| Sand | 0.70 | 0.37 |
| Determined amino acid composition | ||
| Arginine | 1.50 | 0.01 |
| Histidine | 0.57 | 0.00 |
| Isoleucine | 1.00 | 0.01 |
| Leucine | 1.90 | 0.02 |
| Lysine | 1.42 | 0.01 |
| Methionine | 0.59 | 0.00 |
| Phenylalanine | 1.16 | 0.01 |
| Threonine | 0.94 | 0.01 |
| Tryptophan | 0.23 | 0.00 |
| Valine | 1.08 | 0.01 |
| Alanine | 1.09 | 0.01 |
| Aspartic acid | 2.31 | 0.02 |
| Cysteine | 0.34 | 0.00 |
| Glutamic acid | 4.02 | 0.02 |
| Glycine | 0.94 | 0.01 |
| Proline | 1.27 | 0.02 |
| Serine | 0.98 | 0.01 |
Provided per kilogram of DSM Vitamin premix: vitamin A, 2,204,586 IU; vitamin D3, 200,000 ICU; vitamin E, 2,000 IU; vitamin B12, 2 mg; biotin, 20 mg; menadione, 200 mg; thiamine, 400 mg; riboflavin, 800 mg; d-pantothenic acid, 2,000 mg; vitamin B6, 400 mg; niacin, 8,000 mg; folic acid, 100 mg; and choline, 34,720 mg.
Provided per kilogram of mineral premix: Ca, 0.72 g; Mn, 3.04 g; Zn, 2.43 g; Mg, 0.61 g; Fe, 0.59 g; Cu, 22.68 g; I, 22.68 g; and Se, 9.07 g.
Intestinal Morphology
At the end of the study, all birds were euthanized by cervical dislocation. Five centimeters long middle sections of jejunum were collected from one bird per cage. Before fixing intestinal tissue in 10% formalin, digesta were flushed out from the tissue using phosphate buffer saline. Afterward, the fixed intestinal tissues were embedded in paraffin, and 4-μm tissue slides were made and stained with hematoxylin and eosin. The intestinal morphology of each slide was observed and captured by a light microscope with 2X magnification (BZ-X800, Keyence Inc, Itasca, IL).
Ileal Digestibility and Endogenous Loss of Amino Acids
At 6 d postinfection, one-half of the ileum proximal to the ileocecal junction was collected, and the digesta were gently flushed out from the ileum with deionized water. The digesta samples were pooled within the cage, placed on the ice during sample collection, and frozen at −20°C until further analyses. Frozen samples were lyophilized and ground by a coffee grinder (KitchenAid, Benton Harbor, MI). Due to the limited digesta collected from the Medium and High doses-Eimeria-challenged birds, ground samples were further pooled within 2 replicate cages in a ratio of 1 to 1 to gain sufficient samples per replicate for measurement of crude protein, amino acids, and titanium dioxide; therefore, each treatment had four replicates in the present study for the ileal digestibility. Titanium dioxide was analyzed according to the method described by Short et al. (1996). The analysis of amino acids in the digesta and feed was done at the University of Missouri Agricultural Experiment Station Chemical Laboratory [method 982.30 E (a, b, c), AOAC, 2006].
Calculation, Lesion Scores, and Statistical Analysis
Ileal endogenous loss, AID, and SID of amino acids were calculated by the following formulas (Stein et al., 2007).
Where Tii represents the level of titanium in the diet; Tio represents the concentration of titanium in the ileal digesta; Ni represents the determined value of crude protein or amino acids in the diet; No represents the concentration of crude protein or amino acids in the ileal digesta; *measured by the regular diet; #measured by the NFD diet.
All data were analyzed using the GLM program of SAS software (SAS Institute Inc., Cary, NC), and the statistical significance was set at P < 0.05 in the study. The orthogonal polynomial contrasts were conducted to compare the difference between the control treatment and the average of Eimeria-challenged groups and to evaluate the linear and quadratic effects of graded E. maxima challenge on ileal digestibility and endogenous loss of amino acids. Intestinal lesions were scored by the four scale-guideline described by Johnson and Reid (1970). The Kruskal-Wallis nonparametric and post hoc analyses (Elliott and Hynan, 2011) were used for comparing lesion scores among treatments.
RESULTS AND DISCUSSION
The effects of graded infection severity of E. maxima on growth performance, intestinal permeability, and gene expression of tight junction proteins were reported by Teng et al. (2021). The current study further investigated the impacts of increasing challenge doses of E. maxima on endogenous loss and nutrient digestibility of amino acids in broiler chickens. The results showed a significant difference in amino acids concentration in the ileal content between the nonchallenged and Eimeria-challenged groups (Table 2, P < 0.001). Additionally, the levels of amino acids in the digesta were linearly increased in response to the graded challenge doses (Table 2, P < 0.001). The total amino acid values were increased by 7.37, 24.3, and 18.39% in the Low, Medium, and High treatments compared to the Control, respectively. The increase of amino acids in the ileal digesta suggested that coccidiosis reduced nutrient digestibility and increased endogenous losses in broiler chickens.
Table 2.
Effects of graded E. maxima challenge on amino acids composition in the ileal digesta of chickens fed the corn/soybean meal diet at d 6 postinfection (d 20).
| Items1 | Control | Low | Medium | High | SEM | Linear | Quadratic | Control vs. Challenge |
|---|---|---|---|---|---|---|---|---|
| Total | 13.37 | 20.74 | 37.67 | 31.76 | 2.71 | <0.001 | 0.029 | <0.001 |
| Essential amino acids | ||||||||
| Arginine | 0.58 | 1.06 | 2.07 | 1.73 | 0.17 | <0.001 | 0.044 | <0.001 |
| Histidine | 0.33 | 0.65 | 1.32 | 1.13 | 0.11 | <0.001 | 0.036 | <0.001 |
| Isoleucine | 0.62 | 1.00 | 1.89 | 1.57 | 0.14 | <0.001 | 0.031 | <0.001 |
| Leucine | 1.13 | 1.65 | 3.14 | 2.63 | 0.23 | <0.001 | 0.062 | <0.001 |
| Lysine | 0.68 | 1.34 | 2.81 | 2.33 | 0.24 | <0.001 | 0.003 | <0.001 |
| Methionine | 0.15 | 0.31 | 0.69 | 0.54 | 0.06 | <0.001 | 0.028 | <0.001 |
| TSAA | 0.50 | 0.88 | 1.65 | 1.35 | 0.12 | <0.001 | 0.009 | <0.001 |
| Phenylalanine | 0.66 | 1.01 | 1.93 | 1.61 | 0.14 | <0.001 | 0.041 | <0.001 |
| Threonine | 0.72 | 1.16 | 1.99 | 1.67 | 0.14 | <0.001 | 0.012 | <0.001 |
| Tryptophan | 0.14 | 0.20 | 0.29 | 0.25 | 0.02 | <0.001 | 0.004 | <0.001 |
| Valine | 0.81 | 1.32 | 2.50 | 2.07 | 0.19 | <0.001 | 0.024 | <0.001 |
| Nonessential amino acids | ||||||||
| Alanine | 0.68 | 1.11 | 2.17 | 1.81 | 0.17 | <0.001 | 0.042 | <0.001 |
| Aspartic acid | 1.52 | 2.12 | 3.58 | 3.07 | 0.23 | <0.001 | 0.034 | <0.001 |
| Cysteine | 0.36 | 0.58 | 0.95 | 0.82 | 0.06 | <0.001 | 0.002 | <0.001 |
| Glutamic acid | 1.79 | 2.70 | 4.61 | 4.00 | 0.32 | <0.001 | 0.037 | <0.001 |
| Glycine | 0.73 | 1.06 | 1.75 | 1.51 | 0.11 | <0.001 | 0.019 | <0.001 |
| Proline | 0.84 | 1.22 | 2.04 | 1.73 | 0.13 | <0.001 | 0.017 | <0.001 |
| Serine | 0.65 | 0.96 | 1.61 | 1.36 | 0.10 | <0.001 | 0.015 | <0.001 |
The study evaluated the effects of graded challenge of E. maxima on apparent and standard ileal digestibility of amino acids. Chickens were challenged with E. maxima on 14.
Intestinal digesta was collected on 6 d postinfection.
Unit: %, grams per 100 grams of sample.
Abbreviation: TSAA, total sulfur amino acids.
Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima; Control vs. Challenge, nonchallenged control vs. average of all challenged groups.
Increasing severity of Eimeria infection linearly decreased AID of amino acids, energy, and minerals of chickens (Rochell et al., 2016; Teng et al., 2020a). Two main factors that might contribute to the reduction of AID during coccidiosis are 1) endogenous losses from sloughed epithelial cells, enzymes, and mucin, and 2) the increase of undigested dietary nutrients. The brown and yellow area in Figure 1A illustrated that graded severity of Eimeria infection might linearly increase amino acids concentration in the ileal contents, consequently reducing AID of amino acids in chickens.
Coccidiosis causes an enormous increase in sloughed cells within the intestine. Our previous study indicated that Eimeria infection significantly reduced villi height in the duodenum, jejunum, and ileum (Teng et al., 2020a,b). Moreover, Eimeria infection induces intestinal mucin production that may also increase the endogenous loss of amino acids in the ileal digesta (Collier et al., 2008). Apart from the endogenous losses from the intestine, the other proportion of increased amino acids in the ileal digesta may be accumulated from the undigested diet. During Eimeria infection, dietary nutrients were not completely digested or absorbed by chickens; thus, undigested nutrients eventually increased the concentration of amino acids in the lower intestine (Table 2). The malabsorption might be related to the reduced efficacy of nutrient transportation from the intestinal lumen into the intestinal epithelial cells, because Eimeria infection downregulated gene expression of nutrient transporters located at the brush border of the intestine (Paris and Wong, 2013; Su et al., 2015; Teng et al., 2021). Additionally, poor nutrient digestion is common in the Eimeria-infected birds, as the activity of intestinal enzymes, such as sucrase and maltase, are suppressed by coccidiosis (Major and Ruff, 1978; Allen, 1987; Adams et al., 1996). Although there was no direct evidence indicating that coccidiosis inhibits endogenous protease activity, a previous study reported the reduced protein digestion of Eimeria-challenged birds from 2 to 6 d postinfection, compared to the nonchallenged control and the nonchallenged pair-fed groups (Adams et al., 1996). The failure to digest and absorb dietary nutrients hereby increases amino acids in the distal intestine and reduces AID during coccidiosis. Moreover, the increased remnant amino acids in the lower intestine might further induce pathogen infection, such as Clostridium perfringens, consequently promoting the onset of necrotic enteritis (Collier et al., 2008).
In the current study, AID of amino acids were reduced linearly in response to the increase of Eimeria challenge doses (Table 3, P < 0.001). Similarly, the rise of E. acervulina infection severity linearly reduced the apparent digestibility of amino acids (Rochell et al., 2016). Our previous study also demonstrated linearly decreased digestibility of energy in response to the increase in mixed Eimeria spp. infection (Teng et al., 2020a). All these studies showed consistent results that the worst nutrient digestibility was observed in the second-high challenge treatments instead of the highest challenge groups. These findings might be associated with the crowding effects described by Williams (2001). When the host is infected with a massive number of oocysts, the Eimeria spp. will compete for the limited resources in the chicken intestine. Once the space and nutrients were not enough for parasite replication, the crowding effects may moderate the pathogenicity of Eimeria (Williams, 2001).
Table 3.
Effects of graded E. maxima challenge on apparent ileal digestibility of amino acids in chickens fed the corn/soybean meal diet at d 6 postinfection (d 20).
| Items1 | Control | Low | Medium | High | SEM | Linear | Quadratic | Control vs. Challenge |
|---|---|---|---|---|---|---|---|---|
| ———————————–(%)——————————————————— | ||||||||
| Total | 84 | 55 | 5 | 24 | 10 | 0.003 | 0.102 | 0.003 |
| Essential amino acids | ||||||||
| Arginine | 89 | 65 | 10 | 38 | 9 | <0.001 | 0.025 | <0.001 |
| Histidine | 84 | 44 | −55 | −5 | 15 | <0.001 | 0.015 | <0.001 |
| Isoleucine | 83 | 51 | −28 | 16 | 12 | <0.001 | 0.015 | <0.001 |
| Leucine | 84 | 58 | −10 | 25 | 10 | <0.001 | 0.022 | <0.001 |
| Lysine | 87 | 54 | −30 | 14 | 13 | <0.001 | 0.016 | <0.001 |
| Methionine | 93 | 75 | 22 | 51 | 8 | <0.001 | 0.021 | <0.001 |
| TSAA | 85 | 54 | −21 | 23 | 11 | <0.001 | 0.006 | <0.001 |
| Phenylalanine | 84 | 57 | −12 | 25 | 11 | <0.001 | 0.015 | <0.001 |
| Threonine | 79 | 40 | −47 | 6 | 13 | <0.001 | 0.005 | <0.001 |
| Tryptophan | 83 | 58 | 8 | 39 | 8 | <0.001 | 0.002 | <0.001 |
| Valine | 79 | 40 | −57 | −2 | 15 | <0.001 | 0.010 | <0.001 |
| Nonessential amino acids | ||||||||
| Alanine | 83 | 50 | −32 | 11 | 13 | <0.001 | 0.017 | <0.001 |
| Aspartic acid | 82 | 55 | −5 | 28 | 9 | <0.001 | 0.009 | <0.001 |
| Cysteine | 71 | 17 | −97 | −27 | 17 | <0.001 | 0.002 | <0.001 |
| Glutamic acid | 88 | 67 | 23 | 46 | 7 | <0.001 | 0.014 | <0.001 |
| Glycine | 79 | 45 | −29 | 14 | 12 | <0.001 | 0.007 | <0.001 |
| Proline | 82 | 53 | −10 | 27 | 10 | <0.001 | 0.008 | <0.001 |
| Serine | 82 | 52 | −13 | 26 | 10 | <0.001 | 0.006 | <0.001 |
The study evaluated the effects of graded challenge of E. maxima on apparent and standard ileal digestibility of amino acids. Chickens were challenged with E. maxima on d 14.
Intestinal digesta was collected on 6 d postinfection.
Abbreviation: TSAA, total sulfur amino acids.
Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima; Control vs. Challenge, nonchallenged control vs. average of all challenged groups.
E. maxima infection caused a tremendous impact on the cysteine digestibility in the current study (Table 3), reducing from 71% (Control) to 17%, −97%, and −27% in the Low, Medium, and High treatment, respectively. Nevertheless, a previous study showed that E. acervulina infection (500,000 and 1,000,000 sporulated oocysts of E. acervulina) merely reduced the digestibility of amino acids within 5% of the control group (Rochell et al., 2016). The difference of Eimeria species might be the primary reason that causes the inconsistency. E. maxima is moderately to highly pathogenic compared to E. acervulina which is moderately pathogenic (Cervantes et al., 2020). Additionally, Eimeria species exhibit strong preference for site-specificity in the intestine of chickens. E. maxima prefer to invade into subepithelial cells of the jejunum and ileum, whereas E. acervulina infects the epithelial cells of the duodenum (Cervantes et al., 2020). Moreover, a meta-analysis reported that, among 7 species, E. maxima has exhibited the most significant impacts on body weight gain of birds (Kipper et al., 2013). Also, a previous study reported a strong correlation between growth performance and ileal nutrient digestibility following graded Eimeria challenge doses (Teng et al., 2020a). In a brief summary, the infection severity, pathogenicity of Eimeria species, site-specific infection in the intestine, and feed type all play important roles in the nutrient digestibility of chickens. However, a further study is still needed to compare the nutrient digestibility of broiler chickens infected with different Eimeria species.
In the current study, the Medium challenge group showed negative values of AID, except arginine, methionine, and tryptophan (Table 3). The negative digestibility means that the remained nutrients in the ileal digesta are greater than those in the diet. Even though the diets were barely digested and absorbed by chickens infected with Eimeria, the value of nutrient digestibility should have been close to zero instead of resulting in a negative number. This finding could happen when extensive endogenous losses were present in the ileal digesta due to critical intestinal damage. Thus, this is a clear evidence that severe Eimeria infection causes tremendous endogenous losses in the intestine.
Without considering the endogenous losses, AID underestimates the true digestibility. Moreover, the underestimation is worse in the infected birds when Eimeria infection significantly increases pathogen-specific endogenous losses (Figure 1A, brown triangle). The observation of negative digestibility in the current study suggested that AID is not a suitable method to represent and estimate accurate digestibility when chickens are infected with E. maxima.
In order to address the drawback of AID, endogenous loss of amino acids were determined by feeding chickens the NFD diet. The current results indicated that glutamic acids and aspartic acids were the predominant amino acids in the endogenous flow regardless of the Eimeria challenge (Table 4). These findings were in agreement with previous reports (Adedokun et al., 2007; Golian et al., 2008). Moreover, the current results showed a significant linear increase of endogenous amino acid flow in response to the increasing infection severity (Table 2, P < 0.05), except tryptophan. However, the endogenous loss of amino acids in the Low challenge treatment were numerically less than the control group. Similar results were observed in a previous research. Adedokun et al. (2016) reported that mild infection of coccidiosis (12 × vaccine-challenge) reduced endogenous loss of amino acids. Because the coccidia infection decreased villi height in the chicken intestine, it is likely that the reduced mucosa area has minimized the endogenous losses in the case of mild coccidia infection (Adedokun et al., 2016). In the current study, the Medium and High treatments had severely damaged the chicken intestine for an extended period of time. Eventually, the increase of sloughing cells during the acute Eimeria infection may lead to the higher endogenous loss of amino acids. This assumption might explain that the increases of endogenous losses at the sampling day (6 d postinfection) were only observed in the Medium and High challenge groups (Table 4).
Table 4.
Effects of graded E. maxima challenge on ileal endogenous amino acid flow at d 6 postinfection (d 20)
| Items1 | Control | Low | Medium | High | SEM | Linear | Quadratic | Control vs. Challenge |
|---|---|---|---|---|---|---|---|---|
| Total | 6,720 | 6,000 | 7,020 | 8,770 | 373 | 0.025 | 0.082 | 0.486 |
| Essential amino acids | ||||||||
| Arginine | 270 | 240 | 280 | 380 | 18 | 0.020 | 0.047 | 0.461 |
| Histidine | 140 | 120 | 140 | 210 | 11 | 0.012 | 0.039 | 0.384 |
| Isoleucine | 300 | 280 | 320 | 410 | 18 | 0.015 | 0.095 | 0.339 |
| Leucine | 470 | 430 | 490 | 640 | 28 | 0.016 | 0.062 | 0.394 |
| Lysine | 380 | 350 | 400 | 570 | 28 | 0.008 | 0.051 | 0.276 |
| Methionine | 90 | 90 | 100 | 130 | 6 | 0.014 | 0.173 | 0.226 |
| TSAA | 270 | 260 | 300 | 360 | 15 | 0.017 | 0.193 | 0.255 |
| Phenylalanine | 280 | 260 | 300 | 390 | 16 | 0.008 | 0.096 | 0.226 |
| Threonine | 450 | 390 | 470 | 570 | 23 | 0.027 | 0.083 | 0.528 |
| Tryptophan | 60 | 60 | 80 | 80 | 4 | 0.124 | 0.867 | 0.242 |
| Valine | 490 | 430 | 500 | 640 | 27 | 0.027 | 0.045 | 0.614 |
| Nonessential amino acids | ||||||||
| Alanine | 310 | 280 | 330 | 460 | 22 | 0.006 | 0.047 | 0.272 |
| Aspartic acid | 610 | 550 | 630 | 810 | 35 | 0.020 | 0.067 | 0.451 |
| Cysteine | 180 | 170 | 200 | 230 | 9 | 0.021 | 0.212 | 0.281 |
| Glutamic acid | 710 | 640 | 740 | 1030 | 50 | 0.012 | 0.463 | 0.382 |
| Glycine | 340 | 310 | 360 | 460 | 19 | 0.014 | 0.102 | 0.319 |
| Proline | 360 | 330 | 370 | 470 | 20 | 0.043 | 0.096 | 0.564 |
| Serine | 390 | 350 | 410 | 500 | 20 | 0.021 | 0.079 | 0.464 |
The study evaluated the effects of graded challenge of E. maxima on apparent and standard ileal digestibility of amino acids. Chickens were challenged with E. maxima on day 14.
Intestinal digesta was collected on 6 d postinfection.
Abbreviation: TSAA, total sulfur amino acids.
Unit: mg/kg of DM intake.
Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima; Control vs. Challenge, nonchallenged control vs. average of all challenged groups;.
In the current results, the graded challenge of E. maxima linearly decreased the SID of amino acids (Table 5, P < 0.01). However, there was not much difference between the AID and SID. After standardization, digestibility of total amino acid was increased by 5, 3, 2, and 3% in the control, Low, Medium, and High treatments, respectively. Moreover, the challenged birds had 3.3 and 3.7% increases in the SID of threonine and serine compared to the AID, whereas the other amino acids increased less than 3% by the standardization. The higher corrected levels of threonine and serine might be associated with the mucin stimulation caused by coccidiosis. Previous studies have showed that Eimeria infection induced intestinal mucin production and the mucin is rich in threonine, serine, and proline (Lien et al., 1997; Lang et al., 2007; Collier et al., 2008). Furthermore, the mucin is also known to produce protective gel-like layer on the intestinal epithelium against pathogen infection, which may also reduce dietary nutrients absorption from the intestinal lumen (Boegh and Nielsen, 2015; Schroeder, 2019). These findings suggest that coccidiosis induces mucinogenosis and increases the endogenous loss of amino acids.
Table 5.
Effects of graded E. maxima challenge on standard ileal digestibility of amino acids at d 6 postinfection (d 20).
| Items1 | Control | Low | Medium | High | SEM | Linear | Quadratic | Control vs. Challenge | |
|---|---|---|---|---|---|---|---|---|---|
| ———————————–(%)——————————————— | |||||||||
| Total | 89% | 58% | 7% | 27% | 10% | 0.003 | 0.088 | 0.003 | |
| Essential amino acids | |||||||||
| Arginine | 94% | 68% | 12% | 41% | 9% | <0.001 | 0.021 | <0.001 | |
| Histidine | 88% | 46% | −54% | -3% | 16% | <0.001 | 0.013 | <0.001 | |
| Isoleucine | 88% | 54% | −26% | 19% | 12% | <0.001 | 0.014 | <0.001 | |
| Leucine | 88% | 60% | −8% | 28% | 11% | <0.001 | 0.021 | <0.001 | |
| Lysine | 93% | 56% | −29% | 17% | 13% | <0.001 | 0.014 | <0.001 | |
| Methionine | 99% | 78% | 24% | 54% | 8% | <0.001 | 0.017 | <0.001 | |
| TSAA | 90% | 57% | −19% | 26% | 12% | <0.001 | 0.005 | <0.001 | |
| Phenylalanine | 89% | 60% | -10% | 28% | 11% | <0.001 | 0.014 | <0.001 | |
| Threonine | 85% | 43% | -44% | 10% | 14% | <0.001 | 0.004 | <0.001 | |
| Tryptophan | 88% | 61% | 11% | 42% | 8% | <0.001 | 0.002 | <0.0001 | |
| Valine | 85% | 43% | −55% | 1% | 15% | <0.001 | 0.009 | <0.001 | |
| Nonessential amino acids | |||||||||
| Alanine | 87% | 53% | −31% | 14% | 13% | <0.001 | 0.015 | <0.001 | |
| Aspartic acid | 86% | 58% | −3% | 31% | 10% | <0.001 | 0.008 | <0.001 | |
| Cysteine | 76% | 20% | −95% | -24% | 18% | <0.001 | 0.001 | <0.001 | |
| Glutamic Acid | 92% | 70% | 24% | 49% | 7% | <0.001 | 0.012 | <0.001 | |
| Glycine | 83% | 48% | −27% | 18% | 12% | <0.001 | 0.006 | <0.001 | |
| Proline | 86% | 56% | −8% | 30% | 10% | <0.001 | 0.007 | <0.001 | |
| Serine | 88% | 56% | −10% | 30% | 10% | <0.001 | 0.005 | <0.001 | |
The study evaluated the effects of graded challenge of E. maxima on apparent and standard ileal digestibility of amino acids. Chickens were challenged with E. maxima on d 14.
Intestinal digesta was collected on 6 d postinfection.
Abbreviation: TSAA, total sulfur amino acids.
Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima; Control vs Challenge, nonchallenged control vs. average of all challenged groups.
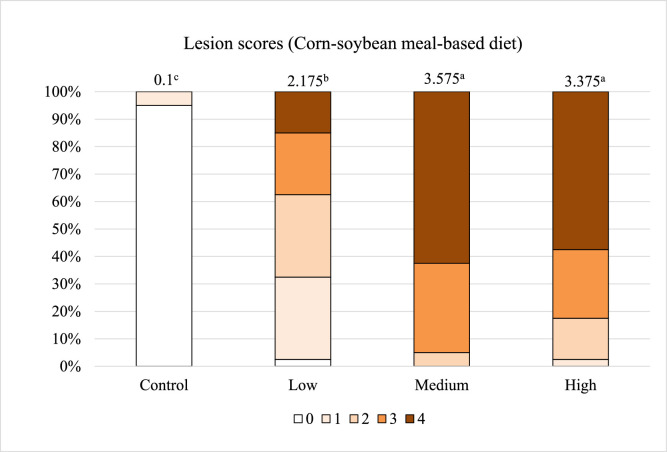
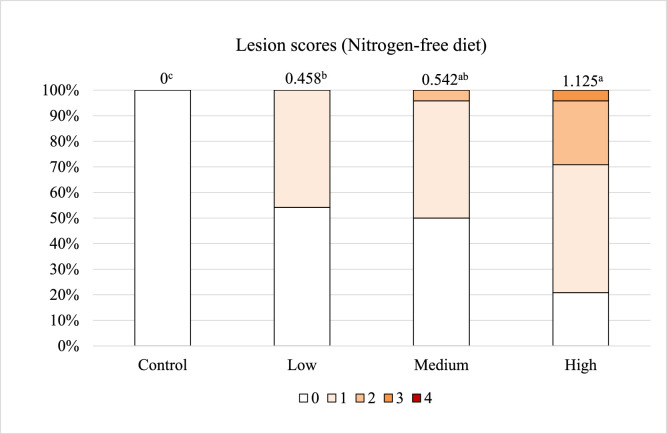
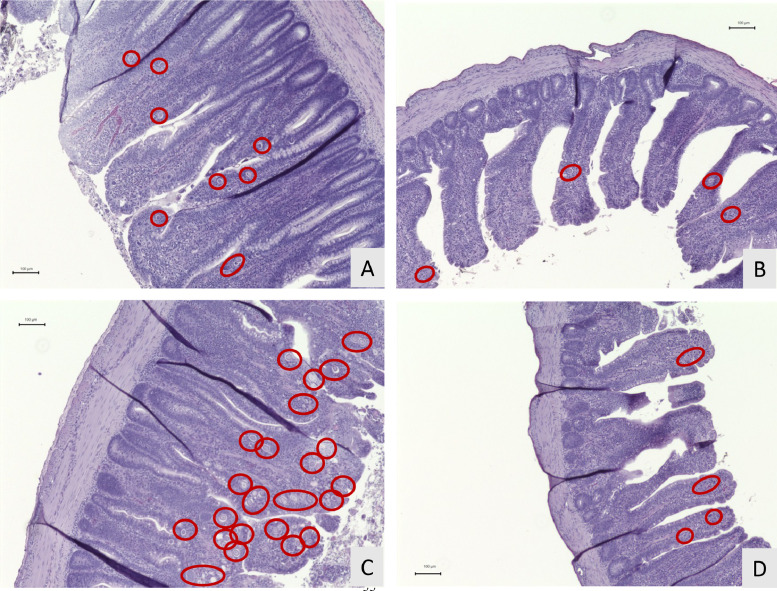
After correcting the endogenous loss, the SID of amino acids should accurately demonstrate how much dietary amino acids will be digested and absorbed by the gastrointestinal tract when chickens are infected with pathogens. However, in the current study, the negative values of SID were present in the Medium challenge group, suggesting that underestimation of endogenous losses has not been completely addressed. We speculated that the endogenous losses determined by the NFD method might be less than that from the birds fed the corn-soybean meal diet, because NFD may negatively impact parasite's development in the host. Ravindran (2021) emphasized that birds fed diets without protein should not be considered physiologically normal, because the NFD diet dramatically impacts body protein synthesis and amino acid balance. Thus, the current study evaluated intestinal lesions from both groups of birds (regular diet and NFD diet) to determine if the dietary factor would influence the Eimeria infection severity. The results showed that the birds fed both diets exhibited increased lesion scores in response to the graded challenge doses (Figure 2, Figure 3, P < 0.01), however the lesion scores in the NFD group were not comparable to those of regular diet. In the High dose treatment, chickens fed the regular diet had the average lesion scores of 3.375, with 58% of the chickens scored with the most severe lesion (score 4), whereas the birds fed the NFD had an average score of 1.125 with 0% of the population evaluated as the highest lesion score. Additionally, the chickens fed the regular diet in the Low challenge treatment even had higher lesion scores than those chickens fed the NFD in the High challenge treatment (2.175 vs. 1.125). Moreover, pictures of intestinal morphology confirm that NFD did not cause as severe infection as the corn-soybean meal-based diet did. Obvious destructed tissue was captured in the Eimeria-infected birds fed the regular diet (Figures 4A and 4C), but only a few gametocytes and oocysts were observed in the NFD groups (Figures 4B and 4D). There was a noticeable difference of intestinal damage between the Low and High challenge groups fed the regular diet (Figures 4A and 4C). The High challenge treatment resulted in acute intestinal destruction with more than 20 parasites per villus, whereas villus shapes remained intact in the Low treatment. In contrary, among the groups fed the NFD, there was not much difference in the numbers of parasites and the magnitude of intestinal destruction. These findings confirm our assumption that NFD can impact parasite development and their pathogenicity. The effects of protein levels on susceptibility to coccidiosis were associated with intestinal trypsin activity (Britton et al., 1964). An in vitro study reported that trypsin and chymotrypsin play crucial roles in excystation of Eimeria sporozoites (Chapman, 1978). A previous study also indicated that gradually reducing protein levels in diet from 20 to 0%, causes decreased host mortality and cecal lesion scores of birds infected with E. tenella (Britton et al., 1964). Similarly, another study reported that reduced mortality and oocyst production were related to decreased dietary crude protein (Sharma et al., 1973). Based on these evidences, NFD might inhibit the excystation of sporocysts by reducing trypsin secretion in the upper intestine. As the NFD failed to cause the same levels of Eimeria infection as the regular diet in the current study, it is suggested that the NFD method underestimates the true endogenous loss of amino acids in Eimeria-infected chickens fed with regular corn-soybean meal-based diet and results in an unexpected influence on the accuracy of SID.
Figure 2.
Effects of graded E. maxima infection on intestinal lesion scores of broiler chickens fed the corn-soybean meal diet. N = 32 birds. Intestinal lesions were scored by the four scale-guideline described by Johnson and Reid (1970). Means with different letters are significantly different (P < 0.05). The study evaluated the effects of graded challenge of E. maxima on endogenous losses, apparent, and standard ileal digestibility in the broiler chickens. Birds were challenged with E. maxima on d 14. Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima.
Figure 3.
Effects of graded E. maxima infection on intestinal lesion scores of broiler chickens fed the nitrogen-free diet. N = 32 birds. Intestinal lesions were scored by the four scale-guideline described by Johnson and Reid (1970). Means with different letters are significantly different (P < 0.05). The study evaluated the effects of graded challenge of E. maxima on endogenous losses, apparent, and standard ileal digestibility in the broiler chickens. Birds were challenged with E. maxima on d 14. Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima.
Figure 4.
Effects of graded E. maxima infection on intestinal morphology of broiler chickens. (A) Challenge with the Low dose and fed the corn-soybean meal diet; (B) challenge with the Low dose and fed the nitrogen-free diet; (C) challenge with the High dose and fed the corn-soybean meal diet; (D) challenge with the high dose and fed the nitrogen-free diet. Macrogametocytes and oocysts are circled in the figure. The study evaluated the effects of graded challenge of E. maxima on endogenous losses, apparent, and standard ileal digestibility in the broiler chickens. Birds were challenged with E. maxima on d 14. Low, 12,500 oocysts of E. maxima; Medium, 25,000 oocysts of E. maxima; High, 50,000 oocysts of E. maxima.
Several other approaches have been used to evaluate the endogenous loss of noninfected chickens. The NFD method was concluded to be the most consistent and reliable way for measuring endogenous amino acid flow (Adedokun et al., 2011), but based on the current findings, the NFD caused a significant inhibition against Eimeria replication which might not be suitable for determining endogenous losses in the Eimeria-challenged chickens. The highly digestible protein method, the regression method, and the isotope dilution method are potential approaches that can be tested in future studies. Pilot experiments are recommended to testify whether the severity of Eimeria infection will be influenced by these alternative methods.
In conclusion, chickens fed the NFD inhibited Eimeria infection in the intestine, consequently underestimating the actual endogenous losses and the SID in the birds fed the regular diet. However, the endogenous amino acid flow and lesion scores were still linearly increased in response to the graded Eimeria challenge doses regardless of the dietary effect. Furthermore, increasing infection severity of E. maxima linearly reduced the AID and SID of amino acids. The current findings also suggested that the AID of nutrients could not represent or estimate the true digestibility in the Eimeria-infected birds, mainly because of the massive endogenous losses during coccidiosis. An alternative methodology for standardizing nutrient digestibility in Eimeria-infected birds is needed, because a precise SID value is empirical for poultry nutritionists to formulate nutrient-balanced diets that minimize the nutrient waste caused by coccidiosis.
Acknowledgments
ACKNOWLEDGMENTS
This study was financed in part by a cooperative agreement 58-6040-8-034 from United States Department of Agriculture-Agricultural Research Service. We express our special thanks to Dr. Fuller and all members in Dr. Kim's research group.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Adams C., Vahl H.A., Veldman A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: development of an experimental infection model. Br. J. Nutr. 1996;75:867–873. doi: 10.1079/bjn19960192. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Adeola O., Parsons C.M., Lilburn M.S., Applegate T.J. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult. Sci. 2011;90:1737–1748. doi: 10.3382/ps.2010-01245. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Ajuwon K.M., Romero L.F., Adeola O. Ileal endogenous amino acid losses: response of broiler chickens to fiber and mild coccidial vaccine challenge. Poult. Sci. 2012;91:899–907. doi: 10.3382/ps.2011-01777. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Helmbrecht A., Applegate T.J. Investigation of the effect of coccidial vaccine challenge on apparent and standardized ileal amino acid digestibility in grower and finisher broilers and its evaluation in 21-day-old broilers. Poult. Sci. 2016;95:1825–1835. doi: 10.3382/ps/pew066. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Parsons C.M., Lilburn M.S., Adeola O., Applegate T.J. Endogenous amino acid flow in broiler chicks is affected by the age of birds and method of estimation. Poult. Sci. 2007;86:2590–2597. doi: 10.3382/ps.2007-00096. [DOI] [PubMed] [Google Scholar]
- Allen P.C. Physiological responses of chicken gut tissue to coccidial infection: comparative effects of Eimeria acervulina and Eimeria mitis on mucosal mass, carotenoid content, and brush border enzyme activity. Poult. Sci. 1987;66:1306–1315. doi: 10.3382/ps.0661306. [DOI] [PubMed] [Google Scholar]
- Allen P.C., Fetterer R.H. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002;15:58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . AOAC International Official Methods of Analysis. 18th ed. AOAC Int.; Gaithersburg, MD: 2006. [Google Scholar]
- Boegh M., Nielsen H.M. Mucus as a barrier to drug delivery - understanding and mimicking the barrier properties. Basic. Clin. Pharmacol. Toxicol. 2015;116:179–186. doi: 10.1111/bcpt.12342. [DOI] [PubMed] [Google Scholar]
- Britton W.M., Hill C.H., Barber C.W. A mechanism of interaction between dietary protein levels and coccidiosis in chicks. J. Nutr. 1964;82:306–310. doi: 10.1093/jn/82.3.306. [DOI] [PubMed] [Google Scholar]
- Cervantes, H. M., L. R. McDougald, and M. C. Jenkins. 2020. Pages 1193-1217 in Coccidiosis, Diseases of Poultry, 14th ed. Willey Blackwell. Hoboken, NJ.
- Chapman H.D. Studies on the excystation of different species of Eimeria in vitro. Z. Parasitenkd. 1978;56:115–121. doi: 10.1007/BF00930742. [DOI] [PubMed] [Google Scholar]
- Cobb Vantress. 2018. COBB broiler management guide. Accessed April 2021. http://cobbsa.co.za/wp-content/uploads/2018/03/BROILER-GUIDE-ENG-2018.pdf.
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Elliott A.C., Hynan L.S. A SAS((R)) macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput. Methods Programs Biomed. 2011;102:75–80. doi: 10.1016/j.cmpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Furuya S., Kaji Y. Estimation of the true ileal digestibility of amino acids and nitrogen from their apparent values for growing pigs. Anim. Feed Sci. Tech. 1989;26:271–285. [Google Scholar]
- Golian A., Guenter W., Hoehler D., Jahanian H., Nyachoti C.M. Comparison of various methods for endogenous ileal amino acid flow determination in broiler chickens. Poult. Sci. 2008;87:706–712. doi: 10.3382/ps.2007-00330. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kipper M., Andretta I., Lehnen C.R., Lovatto P.A., Monteiro S.G. Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet. Parasitol. 2013;196:77–84. doi: 10.1016/j.vetpar.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Lang T., Hansson G.C., Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien K.A., Sauer W.C., Fenton M. Mucin output in ileal digesta of pigs fed a protein-free diet. Z. Ernahrungswiss. 1997;36:182–190. doi: 10.1007/BF01611398. [DOI] [PubMed] [Google Scholar]
- Major J.R., Jr., Ruff M.D. Disaccharidase activity in the intestinal tissue of broilers infected with coccidia. J. Parasitol. 1978;64:706–711. [PubMed] [Google Scholar]
- Nyachoti C.M., Lange C.F.M.d., McBride B.W., Schulze H. Significance of endogenous gut nitrogen losses in the nutrition of growing pigs: a review. Can. J. Anim. Sci. 1997;77:149–163. [Google Scholar]
- Paris N.E., Wong E.A. Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poul. Sci. 2013;92:1331–1335. doi: 10.3382/ps.2012-02966. [DOI] [PubMed] [Google Scholar]
- Ravindran V. Progress in ileal endogenous amino acid flow research in poultry. J. Anim. Sci. Biotechnol. 2021;12:5. doi: 10.1186/s40104-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochell S.J., Parsons C.M., Dilger R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and alpha1-acid glycoprotein in broilers. Poult. Sci. 2016;95:1573–1581. doi: 10.3382/ps/pew035. [DOI] [PubMed] [Google Scholar]
- Schroeder B.O. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019;7:3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.D., Fernando M.A., Summers J.D. The effect of dietary crude protein level on intestinal and cecal coccidiosis in chicken. Can. J. Comp. Med. 1973;37:195–199. [PMC free article] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Tech. 1996;59:215–221. [Google Scholar]
- Siriwan P., Bryden W.L., Mollah Y., Annison E.F. Measurement of endogenous amino acid losses in poultry. Br. Poult. Sci. 1993;34:939–949. doi: 10.1080/00071669308417654. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Fuller M.F., Moughan P.J., Sève B., Mosenthin R., Jansman A.J.M., Fernández J.A., de Lange C.F.M. Definition of apparent, true, and standardized ileal digestibility of amino acids in pigs. Livest. Sci. 2007;109:282–285. [Google Scholar]
- Su S., Miska K.B., Fetterer R.H., Jenkins M.C., Wong E.A. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 2015;150:13–21. doi: 10.1016/j.exppara.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Teng P.Y., Choi J., Tompkins Y.H., Lillehoj H.S., Kim W.K. Impacts of increasing challenge with Eimeria maxima on the growth performance and the gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021;52:81. doi: 10.1186/s13567-021-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Yadav S., Castro F.L.S., Tompkins Y.H., Fuller A.L., Kim W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Yadav S., Dos Santos T.S., Fuller A.L., Kim W.K. 2-Nitro-1-propanol improved nutrient digestibility and oocyst shedding but not growth performance of Eimeria-challenged broilers. Poult. Sci. 2020;99:4314–4322. doi: 10.1016/j.psj.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]