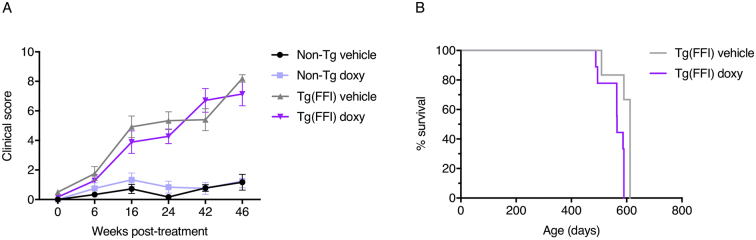
Fig. 5.
Quantitative scores of clinical signs and survival. (A) Clinical signs were rated using objective criteria, each assigned a score (see Section 2). Bars indicate the mean ± SEM of 9 vehicle- and 6 doxy-treated non-Tg, 6 vehicle-treated Tg(FFI-26) and 9 doxy-treated Tg(FFI-26) mice. A linear mixed-effects model with a SP(POW) correlation structure was used. In case of interaction effects, additional Bonferroni's post-hoc tests were done. Three non-Tg and two Tg(FFI-26) mice died of intercurrent, non-neurological illness at 312, 359, 461, 399 and 513 days of age, and were excluded from the analyses. (B) Percentage survival of doxy- and vehicle-treated Tg(FFI-26) mice. The exact Wilcoxon rank-sum test was used. With the exception of three non-Tg mice that died of intercurrent illness, all other non-Tg mice remained healthy and were culled between 587 and 612 days of age.