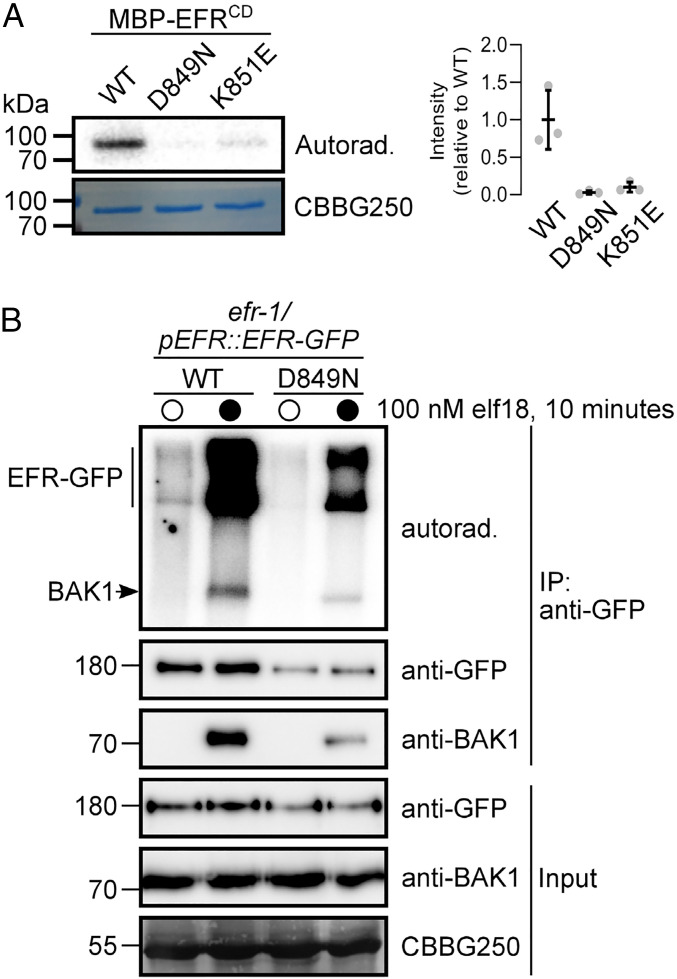
Fig. 1.
EFR is an active protein kinase but its activity is not required for phosphorylation in an isolated receptor complex. (A) In vitro protein kinase activity of recombinant MBP-tagged EFRCD (WT) and catalytic site mutants (D849N and K851E). Recombinant proteins were incubated with 1 µCi γ[32P]ATP for 10 min and 32P incorporation was assessed by autoradiography. Relative quantification of 32P incorporation from three independent assays is shown. (B) On-bead kinase activity assay of immunopurified EFR-GFP (mock treatment, open circles) and EFR-GFP/BAK1 (elf18-treated, closed circles) complexes purified with GFP-Trap beads. Bead-bound receptor complexes were incubated with 5 µCi γ[32P]ATP for 30 min and 32P incorporation was assessed by autoradiography. On-bead kinase activity assays were performed three times with similar results each time. In A and B, Coomassie stain is shown as loading control (CBBG250).