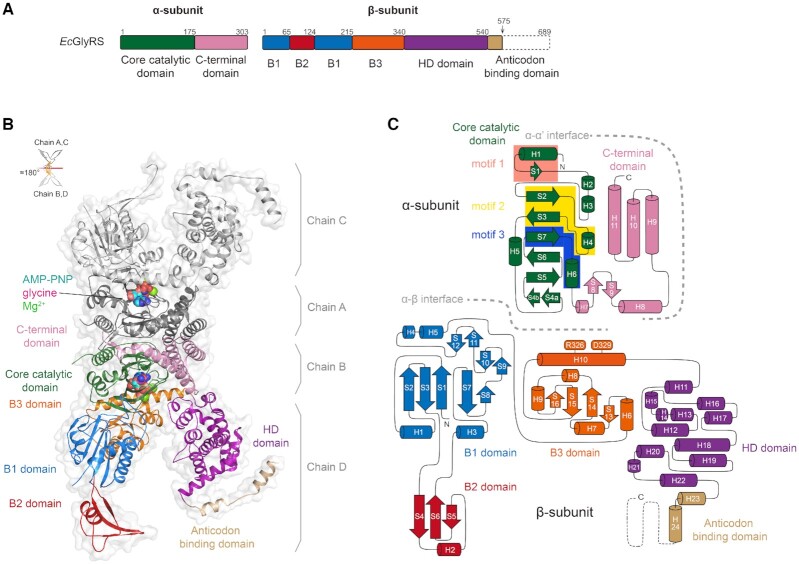
Figure 1.
The overall structure of heterotetrameric EcGlyRS. (A) The domain organization of EcGlyRS. To facilitate crystallization, the C-terminal part of the anticodon binding domain was truncated, resulting in the construct named EcGlyRS575. (B) Cartoon representation of the overall structure of (αβ)2 heterotetrameric EcGlyRS575. The protomer consisting of chains B and D are colored as shown in Figure. 1A, while the other protomer (chains A and C) are colored gray. The glycine, Mg2+, and ATP analog (AMP-PNP) are shown as sphere models in the two aminoacylation pockets. Two protomers are organized through a noncrystallographic 2-fold axis to form the functional heterotetramer. (C) The topology diagram of the α- and β-subunits is colored as depicted in Figure 1A. Arrows represent β-strands, while α- and 310-helices are shown as cylinders. Class II signature motifs 1, 2 and 3 in the α-subunit are highlighted in salmon, yellow and blue, respectively, and the residues Arg326 and Asp329 in the β-subunit B3 domain contributing to ATP binding are labeled.