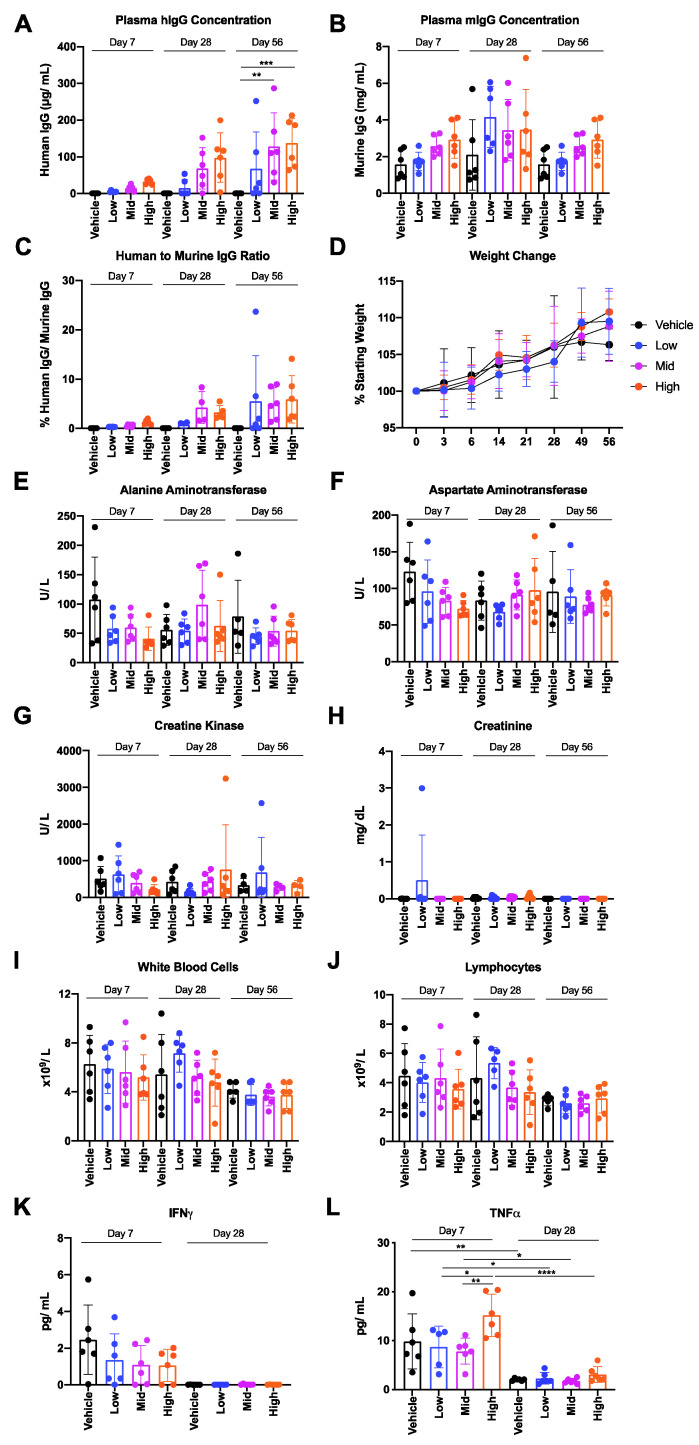
Figure 2.
Findings of the murine AAV6.2FF-31C2 safety and tolerability study. 6-week-old male and female BALB/c mice (n = 6; equal male and female mice per group) were administered either a low (1 × 1011 VG), mid (2 × 1011 VG) or high (6 × 1011 VG) dose and sacrificed either 7-, 28- or 56-days post AAV administration. Terminal blood samples were collected at endpoint and plasma was analyzed for (A) hIgG concentration, (B) mIgG concentration and (C) the ratio of hIgG to mIgG is shown. (D) Mice were weighted on a weekly/ biweekly schedule throughout the in-life phase of the study. Plasma and whole blood samples were analyzed for (E) alanine aminotransferase, (F) aspartate aminotransferase, (G) creatine kinase, (H) creatinine, (I) white blood cells, (J) lymphocytes, IFNγ (K) and TNFα (L). A one-way ANOVA was used for analysis. Data are represented as the mean ± standard deviation. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.