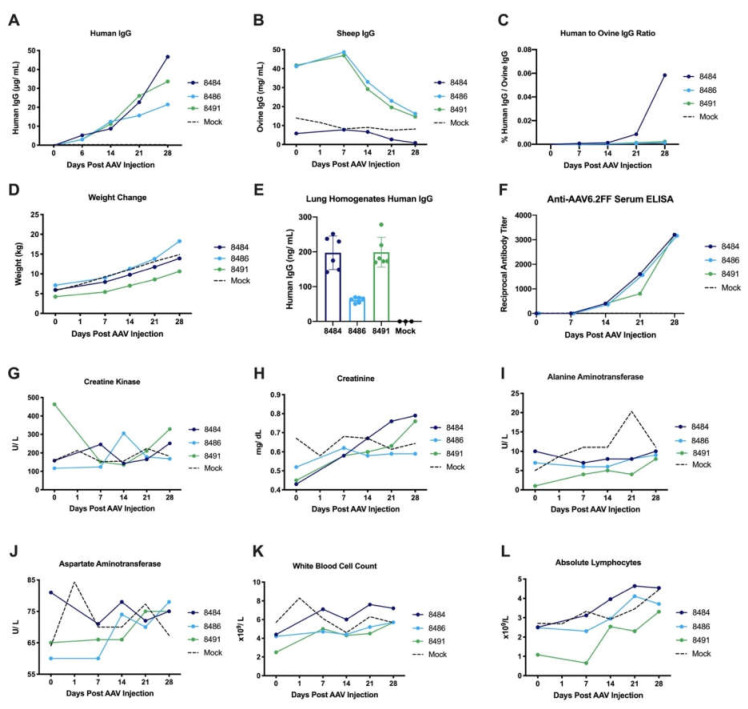
Figure 3.
Findings of the ovine AAV6.2FF-31C2 feasibility study. Three 2-week-old lambs were administered 5x1012 VG/ kg of AAV6.2FF-31C2 by IM injections to the rump. Aged-matched controls (n = 3) were administered PBS IM. Weekly blood samples were collected and analyzed for plasma (A) hIgG concentration, (B) oIgG concentration and (C) the calculated ratio of hIgG to oIgG is displayed. (D) Sheep were weighted weekly throughout the in-life phase of the study. (E) Lung homogenates at endpoint (two sample sites per animal) were analyzed for hIgG concentration. (F) Anti-AAV6.2FF capsid antibodies were monitored weekly. Plasma and whole blood samples were analyzed for (G) creatine kinase, (H) creatinine, (I) alanine aminotransferase, (J) aspartate aminotransferase, (K) white blood cells and (L) absolute lymphocytes. The black dotted line represents the average value for the three control sheep.