Abstract
Equine recurrent uveitis (ERU) causes painful inflammatory attacks and oftentimes blindness in the affected eyes. The disease is considered a late sequela of systemic leptospirosis. The most effective therapy is the surgical removal of the vitreous (vitrectomy), which is not only therapeutic, but provides vitreous material that can be assessed diagnostically. For example, the lipL32 gene, culturable Leptospira spp., and anti-Leptospira antibodies have all been detected in vitreous samples obtained from eyes with chronic ERU. Despite this clear evidence of leptospiral involvement, the systemic administration of antibiotics in infected horses is ineffective at resolving ERU. This syndrome of chronic recurrent inflammation, which is unresponsive to antibiotic therapy, combined with apparent bacteria evading the immune response, is consistent with a biofilm-associated infection. The purpose of this study, therefore, was to detect the in vivo biofilm formation of Leptospira spp. in vitreous samples collected during vitrectomy and examined using a Warthin-Starry silver stain and immunohistochemistry. All known steps of biofilm formation were visualized in these samples, including individual Leptospira spp., leptospiral microcolonies and dense roundish accumulations of Leptospira spp. In many instances spirochetes were surrounded by an extracellular substance. Taken together, data from the present study show that ERU is a biofilm-associated intraocular leptospiral infection, which best explains the typical clinical course.
Keywords: in vivo biofilm formation, biofilm infection, Leptospira spp., immune tolerance, antibiotic tolerance, equine recurrent uveitis (ERU), vitreous humor, immunohistochemistry, Warthin-Starry silver stain
1. Introduction
Biofilm-associated infections are characterized by persistent and progressive disease in which the inflammatory response surrounding the biofilm plays a significant role [1,2]. Four steps of biofilm formation have been described: (1) single bacteria; (2) the formation of microcolonies; (3) a mature biofilm; and (4) the breakup of biofilm and release of planktonic bacteria [3]. In vitro biofilm formation has been described in detail for Leptospira spp. [4,5,6] and there is evidence of in vivo biofilm formation following experimental infections [7,8].
In horses, recurrent uveitis occurs at unpredictable intervals over a period of many years and usually leads to blindness despite intensive conservative therapy [9,10,11]. Both eyes are affected in about 25–50% of horses [12,13]. ERU affects up to 10% of all horses in Europe [14,15,16] and up to 25% in the US [17].
In European horses without a leopard coat pattern, the most effective method to prevent further episodes of uveitis and thereby preserve vision is vitrectomy [10,18,19,20,21]. After a properly performed vitrectomy, which has been routinely performed for more than 30 years in horses with ERU, the control of inflammation is seen in 90–97% of horses [10,13,21,22,23,24]. By contrast, uveitis in horses with a leopard coat pattern manifests differently. Affected horses typically do not appear to be as painful. Intraocular leptospiral infection is rarely confirmed suggesting that the etiology and pathogenesis are different from that seen in horses without a leopard coat pattern [25].
The vitreous removed during vitrectomy are intensely investigated in the hope of better determining etiology, pathogenesis and treatment strategies for ERU. Many of these studies have suggested an association between leptospiral infection and ERU [26,27,28,29,30,31]. For example, anti-Leptospira antibodies are regularly detected in vitreous material obtained during vitrectomies [13,15,21,32,33,34,35,36,37,38,39,40,41]. These antibodies are detected using the micro agglutination test (MAT), and various enzyme-linked immunosorbent assays (ELISA) [42,43]. In other studies, Leptospira spp. are cultured and anti-Leptospira antibodies are detected in the same vitreous samples [13,21,32,35,39,44,45,46]. In addition, lipL32 gene or 16S-rRNA are detected by a polymerase chain reaction (PCR) in up to 70% of intraocular samples collected from affected horses [32,39,43,44,45].
Finally, scanning electron microscopy is used to reliably detect Leptospira spp. in vitreous material from equine eyes affected with recurrent uveitis. The leptospiral organisms were surrounded by a homogeneous granular layer, which has not been seen in Leptospira spp. cultured in vitro (using standard World Health Organization (WHO) strains) [47,48].
In addition to direct evidence of the leptospiral infection of vitreous samples from eyes affected with ERU, evidence of autoimmunity also exists [49]. However, Prof. Deeg’s research group [50] performed all investigations using vitreous samples, from which leptospiral infections were also regularly detected [13,21,39,41,42,43,46]. Furthermore, while vitrectomy removes the vitreous material, potential autoantigens from other tissues—especially the lens and retina—remain in the eye [18,39] which contradicts the idea of autoimmunity [39,51].
Taken together, data from numerous studies using specimens from eyes affected with ERU support the hypothesis that ERU is triggered and perpetuated by the chronic infection of the vitreous cavity with Leptospira spp., and that this may be eliminated by vitrectomy [13,21,32,33,36,37,39,44]. However, systemic antibiotic therapy has not been successful at controlling the inflammation seen in ERU.
The vitreous body consists of 98–99% water, contains collagen fibrils and represents a 28 mL immunological niche in horses [13,39,52,53,54]. These are optimal conditions for the biofilm production of the Leptospira spp. [55]. In addition, ERU exhibits all of the characteristics of a biofilm infection: chronicity, inflammation, and a high tolerance to both antibiotics [46] and the body’s immune defenses. The aim of this ex vivo study was to demonstrate, using Warthin-Starry silver stain and immunohistochemistry, the biofilm formation by Leptospira spp. in vitreous material obtained during vitrectomies performed on horses with ERU.
2. Materials and Methods
2.1. Positive Controls
Culture Leptospira spp. (WHO strains) were spread on microscope slides (Thermo Scientific Superfrost Ultra Plus; Menzel B.V. & Co.KG, Braunschweig, Germany) and allowed to dry overnight. Warthin-Starry silver stain and immunohistochemistry were then performed. The leptospiral serovars used for this study (Grippotyphosa, Bratislava, Australis, Autumnalis, Icterohaemorrhagiae and Pomona) were obtained from an accredited laboratory (State Office for Health and Food Safety, Oberschleissheim, Germany; accreditation DIN EN ISO 17025, Reg. No.: D-PL-19082-02-00).
2.2. Sample Selection
After careful case selection of horses whose history and clinical findings indicated Leptospira-induced uveitis [21], vitrectomy was performed as a therapeutic procedure at the Equine Clinic of Ludwig-Maximilians-University in Munich, Germany. All vitrectomies were performed as previously described [18]. At the beginning of surgery, about 3 mL of undiluted vitreous humor was obtained routinely from the suction line. In order to confirm intraocular leptospiral infection, an aliquot of each sample was sent to an external laboratory for MAT and PCR (Society for Innovative Veterinary Diagnostics, Seelze-Letter, German accreditation authority: D-PL-18303-02-00). Additionally, some vitreous humor was spread on microscope slides and allowed to dry overnight. Approximately 10 smears were obtained from each vitreous sample from ERU eyes. Only samples with a positive PCR result were used for the examinations (Table 1).
Table 1.
Signalment of equine patients, clinical diagnosis and laboratory findings of vitreous samples.
| Sample | Signalment | Clinical Diagnosis | lipL32 Gene PCR Result (Ct Value) | Vitreal Leptospiral Antibody Titer (MAT) 1 |
|---|---|---|---|---|
| 1 | 4-year-old warmblood gelding | ERU | Positive (Ct 37) | Grippotyphosa 1:3200 |
| 2 | 8-year-old Icelandic horse mare | ERU | Positive (Ct 40) | Grippotyphosa 1:400 |
| 3 | 7-year-old Friesian mare | ERU | Positive (Ct 29) | Grippotyphosa 1:3200 |
| 4 | 7-year-old warmblood mare | ERU | Positive (Ct 37) | Grippotyphosa 1:800 |
| 5 | 15-year-old warmblood gelding | ERU | Positive (Ct 38) | Grippotyphosa 1:400 |
| 6 | 10-year-old Friesian gelding | ERU | Positive (Ct 30) | Altoduro 1:400 |
| 7 | 6-year-old warmblood mare | ERU | Positive (Ct 32) | Grippotyphosa 1:3200 |
| 8 | 7-year-old warmblood mare | ERU | Positive (Ct 35) | Grippotyphosa 1:3200 |
| 9 | 4-year-old thoroughbred gelding | ERU | Positive (Ct 33) | Grippotyphosa 1:3200 |
| 10 | 6-year-old warmblood mare | ERU | Positive (Ct 34) | Grippotyphosa 1:3200 |
| 11 | 5-year-old Icelandic horse gelding | ERU | Positive (Ct 33) | Altoduro 1:100 |
| 12 | 5-year-old warmblood stallion | ERU | Positive (Ct 33) | Grippotyphosa 1:3200 |
| 13 | 5-year-old warmblood gelding | ERU | Positive (Ct 38) | Grippotyphosa 1:3200 |
| 14 | 10-year-old warmblood gelding | ERU | Positive (Ct 39) | Grippotyphosa 1:800 |
| 15 | 8-year-old warmblood mare | ERU | Positive (Ct 38) | Grippotyphosa 1:800 |
| 16 | 5-year-old warmblood mare | ERU | Positive (Ct 35) | Grippotyphosa 1:3200 |
| 17 | 8-year-old warmblood gelding | ERU | Positive (Ct 35) | Grippotyphosa 1:200 |
| 18 | 5-year-old warmblood gelding | ERU | Positive (Ct 32) | Grippotyphosa 1:200 |
| 19 | 5-year-old warmblood mare | ERU | Positive (Ct 33) | Grippotyphosa 1:1600 |
| 20 | 4-year-old warmblood gelding | ERU | Positive (Ct 33) | Grippotyphosa 1:100 |
| 21 | 3-year-old warmblood mare | ERU | Positive (Ct 38) | Negative |
| 22 | 10-year-old warmblood gelding | ERU | Positive (Ct 36) | Grippotyphosa 1:3200 |
| 23 | 6-year-old warmblood mare | ERU | Positive (Ct 36) | Grippotyphosa 1:3200 |
| 24 | 7-year-old purebred Spanish stallion | ERU | Positive (Ct 39) | Grippotyphosa 1:3200 |
| 25 | 10-year-old purebred Spanish mare | ERU | Positive (Ct 39) | Australis 1:3200 |
| 26 | 15-year-old warmblood mare | ERU | Positive (Ct 39) | Grippotyphosa 1:3200 |
| 27 | 8-year-old Welsh pony mare | ERU | Positive (Ct 32) | Grippotyphosa 1:3200 |
| 28 | 7-year-old warmblood gelding | ERU | Positive (Ct 29) | Pomona 1:3200 |
| 29 | 12-year-old warmblood gelding | ERU | Positive (Ct 35) | Grippotyphosa 1:3200 |
| 30 | 8-year-old warmblood gelding | Normal 2 | Negative | Negative |
| 31 | 23-year-old Haflinger mare | Normal 2 | Negative | Negative |
| 32 | 17-year-old warmblood gelding | Normal 2 | Negative | Negative |
1 Only the serovar with the highest titer is shown. 2 Clinical examinations revealed no signs of ERU.
2.3. Negative Controls
For subsequent analysis using Warthin-Starry silver stain and immunohistochemistry, vitreous humor samples from clinically normal eyes in which anti-Leptospira antibodies could not be detected by MAT and the lipL32 gene was not detected after 40 cycles of qPCR were used as controls (Table 1).
2.4. Warthin-Starry Silver Stain
A modified Warthin-Starry [56] protocol was used to identify leptospiral organisms in vitreous humor samples. The slides were first incubated in 1% silver nitrate solution (Fa. Morphisto, Offenbach a. M., Germany) for half an hour at 60 °C in the dark and then briefly rinsed in distilled water. A solution containing hydroquinone (“silver enhancer stock solution B” Fa. Morphisto, Offenbach a. M., Germany) was used to reduce the bound silver to a visible metallic form. Developer solution was prepared and preheated to 55–60 °C in a water bath and afterwards placed onto the slides for 2–3 min. The developing process was performed under visual control. The slides were briefly drained and rinsed twice for 1 min. each in warm distilled water (55–60 °C), incubated with 5% sodium thiosulfate (Fa. Morphisto, Offenbach a. M., Germany) at room temperature, and then rinsed for 3 min. under running tap water. Finally, the smears were covered with eukitt® (Fa. Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) for microscopic evaluation.
2.5. Immunohistochemistry
The slides were pulled through the flame twice for heat fixation. All washing steps were done with phosphate buffered saline (PBS) (pH 7.4), and all incubation steps took place at room temperature. After washing once for 4 min, non-specific binding was reduced by bathing in regular protein block (Dako Protein-Block-Serum-Free, Dako GmbH, Jena, Germany) for 5 min. The slides were then incubated for one hour with a specific antibody (anti-Leptospira rabbit antiserum from the OIE and National Collaborating Centre for Reference and Research on Leptospirosis, Academic Medical Center, Department of Medical Microbiology, University of Amsterdam) diluted 1:5120 in Dako Diluent, Dako GmbH, Jena, Germany). After washing twice for 1 min each time, slides were incubated for 30 min. with secondary antibody (Biotinylated anti-rabbit IgG, Biozol, Eching, Germany) diluted 1:300. After washing twice more, slides were incubated with horseradish peroxidase conjugate (Streptavidin-HRP, Leika, Germany) for 20 min. After the last wash, slides were incubated for 5 min in hydrogen peroxide solution (DAB, Dako GmbH, Jena, Germany), and washed with PBS and tap water. After dehydrating in alcohol (70% alcohol, 96% alcohol, 2x isopropanol, 2x xylene) slides were covered with eukitt® (Fa. Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) for microscopic evaluation. Cultured Leptospira spp. from an accredited leptospiral laboratory were served as positive tissue controls (State Office for Health and Food Safety, Oberschleissheim, Germany; accreditation DIN EN ISO 17025, Reg.-Nr.: D-PL-19082-02-00). Samples incubated without primary antibodies formed system controls. Vitreous samples from clinically normal eyes served as negative tissue controls (Table 1).
2.6. Microscopy Images Acquisition
The evaluation was performed with a light microscope (Leica DM 5000B) connected to a digital color camera (Leica DFC 450 C). Samples were routinely screened first using the 10×, then the 40×, and finally the 100× objective (with oil). Representative areas were photographed.
The results are aligned according to the steps of biofilm formation already described by Jamal et al. [3]:
-
Initial contact/attachment to the surface:
Adhesion and cohesion; the attachment to a surface, as well as the attachment of bacteria to each other.
-
Microcolony formation:
After stable attachment to a surface or other bacteria, the bacteria began to multiply by cell division, as well as forming extracellular polymeric substances (EPS), which led to the formation of microcolonies.
-
Maturation and architecture:
EPS are the main components of the biofilm. Cell density in the biofilm is controlled by intercellular signaling and cell-to-cell communication (quorum sensing). This leads to the production of EPS and thus to a three-dimensional dense biofilm structure.
-
Detachment/dispersion of biofilm:
Multiplication of bacteria in the biofilm results in further spreading and reattachment to a surface or other bacteria.
3. Results
Twenty-nine PCR-positive vitreous samples were selected from equine eyes clinically diagnosed with ERU (Table 1, samples 1–29) to attempt to visualize the different steps of biofilm formation. As a control, three vitreous samples were obtained from clinically healthy eyes (Table 1, samples 30–32). The vitreal leptospiral antibody titer test was positive in 28 (96.6%) of 29 samples. The negative MAT result of sample no. 21 could be due to the fact that either no antibodies had yet been formed by the time the sample was taken, or antibodies were no longer detectable, or the overall immune response was so low that it was not possible to detect antibodies by MAT.
3.1. Positive Controls
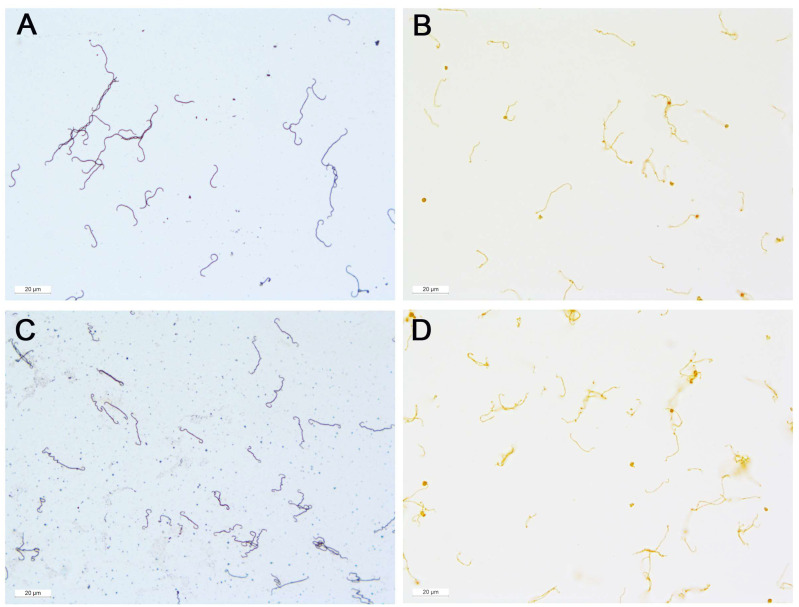
The cultured Leptospira spp. appear as single thin corkscrew-shaped organisms in both Warthin-Starry silver stain and immunohistochemistry, as is typical for this genus. The length of the single bacteria is approximately 10 to 20 µm and they have a very thin diameter of approximately 0.5 µm. They have hooked ends on one or both sides, which distinguishes them from other spirochaetes. The individual bacteria are sometimes completely elongated, generally curved, or have more complex S, U or L shapes. Sometimes one or more bacteria lie side by side, on top of each other, or are intertwined. However, the individual bacterium is always clearly distinguishable. In some cases, the corkscrew-like coiling can be clearly seen as small bumps on the thin, filamentous leptospiral body (Figure 1). No evidence of biofilm formation was found in the cultured Leptospira spp.
Figure 1.
Smears from culture Leptospira spp. (A): Serovar Grippotyphosa, Warthin-Starry stain. (B): Serovar Grippotyphosa, immunohistochemistry. (C): Serovar Australis, Warthin-Starry stain. (D): Serovar Australis, immunohistochemistry.
3.2. Samples from ERU Eyes
Leptospira spp. could not be detected on every slide examined. This can be explained by the small sample volume and the inhomogeneous vitreous matrix. Nevertheless, at least one of the three steps of biofilm formation could be found in smears of each vitreous sample using both staining methods. In addition, it was possible to show all three steps of biofilm formation on the same slides. The evaluation of the slides was extremely time-consuming.
3.3. Steps in Biofilm Formation
Initial contact/attachment to the surface:
Both the Warthin-Starry silver stain (Figure 2A) and immunohistochemistry (Figure 2B) revealed solitary dark brown stained Leptospira spp. Individual Leptospira spp. showed the characteristics of spiral coils and hooked ends as described above, while two or more organisms were sometimes seen lying next to or crossing each other. Regardless, individual organisms could always be distinguished from each other easily (Figure 2A,B).
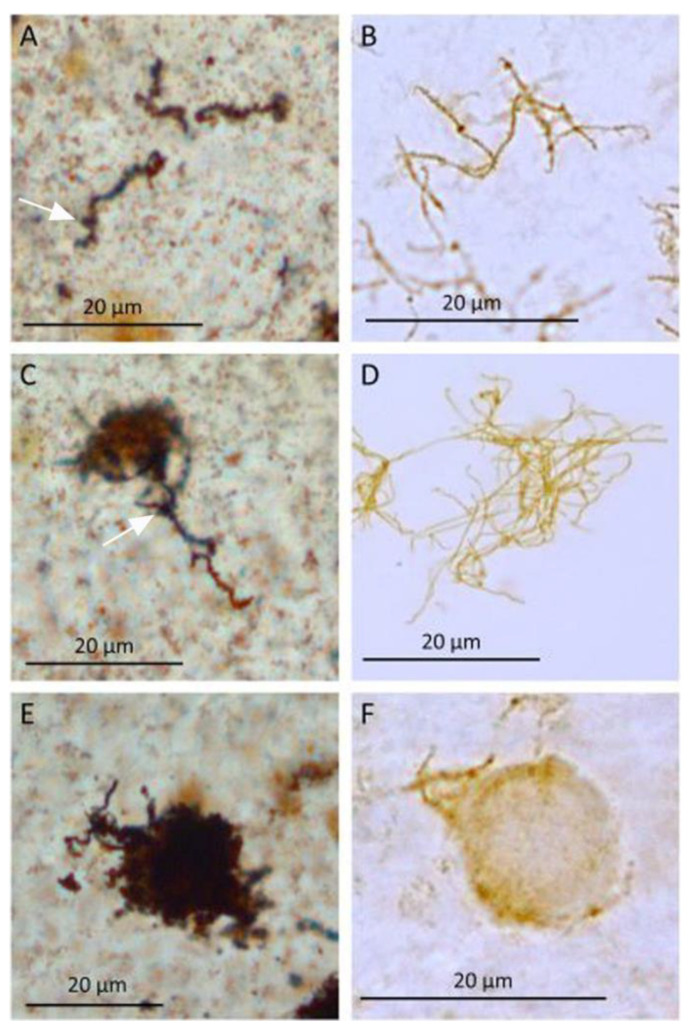
Figure 2.
Smears from vitreous body samples from horses diagnosed with ERU. (A) Solitary Leptospira spp., Warthin-Starry stain. (B) Solitary Leptospira spp., immunohistochemistry. (C) Leptospiral aggregates and surrounding matrix, Warthin-Starry stain. (D) Leptospiral aggregates and surrounding matrix, immunohistochemistry. (E) Dense roundish conglomerates composed of Leptospira spp. and matrix, Warthin-Starry stain. (F) Dense roundish conglomerates composed of Leptospira spp. and matrix, immunohistochemistry. (Arrows: dense round structures on the Leptospira spp.).
Overall, the Leptospira spp. from the vitreous samples of horses affected with ERU appear somewhat thicker and less delicate than the cultured Leptospira spp. (Figure 1). Leptospira spp. from vitreous samples show a significant increase in thickness (approximately 1–2 µm) especially in the Warthin-Starry silver stain, where their shape becomes more blurred. In some cases, Leptospira spp. are covered by granular structures. In addition, a stronger staining of the leptospiral environment can be seen, which is represented by the light brown (IHC) or dark brown (Warthin-Starry silver stain) stained and partially granulated matrix (Figure 2).
-
2.
Microcolony formation:
Individual, well-defined Leptospira spp. and dense variably sized aggregates of Leptospira spp. were seen using either silver stain or the antibody against Grippotyphosa (Figure 2C,D). The aggregates were round, oval or polygonal with some having “frayed” borders consisting of individual intact or fragmental Leptospira spp. (Figure 2C,D). A variably intensely positive, immunohistochemical, reacting homogeneous matrix was evident between the densely packed Leptospira aggregates (Figure 2D). Similarly, in the Warthin-Starry silver stain, a slightly weaker stained inhomogeneous granular matrix appeared between the denser bacteria, which was strongly stained (Figure 2C).
-
3.
Maturation and architecture:
The leptospiral aggregates and the secreted substance condensed such that individual Leptospira spp. could not be distinguished from each other (Figure 2E,F). These condensed structures were approximately round with a diameter of approximately 5 to 20 µm and were clearly distinguishable from the surroundings. Occasionally, individual Leptospira spp. protruding from the aggregates could be identified by their characteristic corkscrew-like coiling and/or hooked ends (Figure 2E,F).
3.4. Negative Controls
Vitreous samples from clinically healthy equine eyes were negative for both leptospiral PCR and leptospiral antibody titers (MAT) (Table 1, sample 30–32). Neither Leptospira spp. nor evidence of biofilm formation were detected in these samples using Warthin-Starry silver stain or immunohistochemistry.
4. Discussion
Descriptions of in vitro biofilm formation have been published for Borrelia spp. [57] and Leptospira spp. [5,6]. In vivo biofilm formation is described in humans with lymphoma [58] or Lyme borreliosis [59]. Although the in vivo biofilm formation by Leptospira spp. has been suspected in renal tubules [5,60], to date it is found exclusively after experimental infections [7,8]. The attachment of bacteria to surfaces is considered to be of great importance for biofilm formation. However, pathogens can also attach to each other and can form a biofilm without being attached to another surface or host tissue. A biofilm can occur in liquid or mucous media [2,3,61,62,63]. The mechanical or surgical removal of the biofilm is described as the best way to eliminate chronic biofilm-associated infections [64,65,66].
To the authors’ knowledge, the present study was the first to detect Leptospira spp. and different steps of biofilm formation in vitreous samples of horses with ERU using immunohistochemical analysis and Warthin-Starry silver staining. Previously, IHC analyses were performed exclusively on fixed tissue sections. In addition, MAT, ELISA, fluorescent antibody tests, dark-field microscopy, culture, PCR, and histopathology with special stains are all used to identify anti-Leptospira antibodies, Leptospira spp., or leptospiral DNA in the tissues or body fluids of dogs [67,68,69]. Whereas, in hamsters, the leptospiral injury of the kidney has been investigated using culture, histology, MAT, serum creatinine concentrations, and immunofluorescent staining [70]. In the present study, tissue sections are not available. Rather, the undiluted vitreous samples of 2–3 mL per patient were utilized. This necessitated the development of a method that does not notably alter spirochete morphology but in which the organisms are reliably stained. Two different techniques are used for this purpose:
Silver plating [71,72,73] is considered a reliable technique for the demonstration of spirochetes. Difficulties with this method arise, however, when only a few organisms are present, since leptospiral fragments cannot be detected. Therefore, in the present study, we used a rabbit anti-Leptospira antibody and a modified immunohistochemical protocol as a supplemental method for the morphological visualization of Leptospira spp. and of the various steps of biofilm formation. IHC has been previously described as a specific detection method of leptospiral antigens in various tissue samples and species and for studying biofilm formation in vivo of pathogenic Leptospira spp. [74,75,76].
To the authors’ knowledge, this is also the first morphological description of the steps of biofilm formation of Leptospira spp. in vivo; however, the same steps of biofilm formation described for bacteria in general [3,64,77] were demonstrated here using Warthin-Starry silver stain and IHC. In addition, the morphological characteristics of in vivo biofilm formation demonstrated in the present study were similar to those shown in vitro for saprophytic and pathogenic Leptospira spp., using transmission electron microscopic studies [5]. Individual Leptospira spp., cell aggregates, microcolonies, and, ultimately, mature biofilm structures were all visualized in the present study. In accordance with Ristow et al. [5], bacterial colonies in the mature biofilm were densely packed, surrounded by an amorphous mass, and formed an approximately round structure.
In vitro, Leptospira spp. are capable of biofilm formation within a few days [5,6] with the first stage requiring cohesion (binding of bacteria to each other) and adhesion (binding of bacteria to a surface) [78,79]. After individual bacteria assemble into microcolonies they surround themselves with an amorphous polymer matrix [62], with the mature biofilm structure taking on a round shape and individual Leptospira spp. no longer recognizable [6]. Because the polymer matrix of the biofilm is produced by the bacteria themselves it is detectable using antibodies directed against Leptospira spp. In the present study, bacterial cohesion was morphologically evident by silver staining and IHC labeling; however, other structures of the vitreous (collagen fibrils, cells, and inflammatory products formed as a result of uveitis) were not stained. IHC was previously described as a specific detection method of leptospiral antigens in various tissue samples and species and for studying biofilm formation in vivo of pathogenic Leptospira spp. [74,75,76].
Canine renal tissues were stained with specific leptospiral antigens and were further investigated with the silver staining method. Therefore, the results of Wild et al. [80] show that immunohistochemistry is a helpful method in the diagnosis of canine leptospirosis. Additionally, immunohistochemistry and the Warthin-Starry stain showed that pathogenic Leptospira spp. were present on the surface of pulmonary epithelium [81].
The slides on which no Leptospira spp. were found can be explained, among other things, by the fact that the vitreous is very inhomogeneous, consists of 98% water, and only about 1 mL of the vitreous material is available undiluted for smears. All other studies detecting Leptospira spp. use tissue guided by other structures and serial sections are available [76,81].
Six criteria have been established to describe biofilm-associated infections: four original criteria by Parsek and Singh [82], and two additional criteria by Hall-Stoodley and Stoodley [83]. It is worthwhile to consider each of these criteria individually as they relate to observations in the present study.
Infecting bacteria are bound to a substrate or a surface [82]:
Although attachment of Leptospira spp. to a surface is not demonstrated in the present study, this is not expected since the vitreous body is composed largely of water. In addition, biofilm formation without attachment to a surface has been described. It is likely in these situations that this occurs via the cohesion of bacteria to each other. For example, a Pseudomonas aeruginosa biofilm without surface attachment is found in tracheobronchial secretions [2,3,61,62]. It is also possible for bacterial adhesion to occur at a microscopic (cellular or subcellular) rather than tissue level, for example, in the vitreous cavity to collagen fibrils [53,54] present in the normal and inflamed vitreous, or to a serum amyloid A [84,85] present in inflamed vitreous. In aquatic environments, plant fibers represent comparable surfaces on which Leptospira spp. biofilm formation can begin [86]. Regardless of whether or not fibrils initially serve as surfaces for the attachment in the vitreous cavity, leptospiral biofilm formation may additionally occur freely in aqueous vitreous phases.
-
2.
The direct examination of infected tissue reveals bacteria living in cell aggregates or microcolonies surrounded by extracellular matrix [82]:
Immunohistochemical examination of vitreous samples from eyes with ERU in the present study reveals various steps of biofilm formation, including cell aggregates and microcolonies. In each case, bacteria are surrounded by an extracellular matrix that is also immunopositive. This extracellular matrix surrounding Leptospira spp. is also found ultrastructurally in vitreous samples from equine eyes affected with ERU [47,48].
-
3.
Biofilm-associated infections are generally confined to a specific location (although bacterial dissemination may occur, it is considered a secondary phenomenon) [82]:
Leptospiral infection in horses with ERU is an intraocular infection exclusively detectable in the aqueous humor and vitreous samples [13,39]. Since both leptospiral culture and PCR are positive more frequently with vitreous material than with the aqueous humor, there is much to suggest that Leptospira spp. persist in the vitreous cavity [13,39]. The detection of Leptospira spp. or its DNA within the aqueous humor may represent organisms that have entered the anterior chamber of the eye from the vitreous humor between the zonular fibers of the lens since there is limited exchange of water between the two humors. By contrast, the persistence of Leptospira spp. in the lens or the highly vascular uvea seems unlikely. Similarly, there is no evidence for the dissemination of Leptospira spp. from the eye to other systemic sites. Further supporting the hypothesis that leptospiral infection is limited to the vitreous is the knowledge that the vitrectomy reliably eliminates ERU [13,18,21,39]. After vitrectomies, intraocular antibody titers decrease continuously and after one year MAT becomes negative, and Leptospira spp. cannot be cultured and neither the lipL32 gene nor 16S rRNA can be detected by PCR in intraocular samples [39].
-
4.
Biofilm-associated infections are impossible or difficult to eliminate using antibiotics, to which the responsible organisms are sensitive when in their planktonic or free-living state [82]:
The systemic administration of antibiotics effective against Leptospira spp. are ineffective at controlling ERU. Despite vitreal enrofloxacin concentrations above the minimum inhibitory concentrations (MIC) for Leptospira spp., these organisms could be cultured from vitreous material [46]. In fact, cultures were only slightly less frequently positive in enrofloxacin-treated (30%) than in untreated (54%) horses. Leptospira spp. have also been cultured in some cases from the irrigation fluid collected during vitrectomies, in which the removed vitreous material is diluted approximately 10-fold and exposed to 0.8 mg/mL of gentamicin [40,87]—approximately 100-fold higher than the MIC [88].
-
5.
No organism can be cultured despite a strong presumption of infection with the pathogen of interest [83]:
Conditions for the culture of Leptospira spp. were demanding. Even with the optimal collection techniques of undiluted vitreous samples and the immediate sterile inoculation into a transport medium, Leptospira spp. were cultured from only 53% of the samples [13,39]. This percentage is comparatively high. However, the positive culture result was often obtained only after several months. In other studies, Leptospira spp. could be cultured in only a small percentage of the vitreous samples examined [32,35,44]. Many other investigators failed to culture Leptospira spp. [26,31,89,90], which led to the hypothesis that ERU is an autoimmune disease [90,91,92].
-
6.
Ineffective immune response as evidenced by bacterial aggregates surrounded by inflammatory cells within host tissue [83]:
The vitreous body is 98%–99% water, but also contains collagen fibrils, occasional cells, and hyaluronic acid [52,53,54]. As such, vitreous material is not a typical “tissue” but rather a somewhat heterogeneous viscous liquid. The histological preparations of vitreous specimens often do not show stainable Leptospira spp. Therefore, an inflammatory reaction surrounding the bacterial aggregates is not present in the same form seen in other tissues. However, when considering the closest vascularized tissue—the uvea, an increase in inflammatory cells is noted, especially in the ciliary body [93,94], but also within the vitreous cavity [47,48,53,95]. Despite high antibody titers and the presence of macrophages within the vitreous, vitreal Leptospira spp. are not eliminated. On the contrary, high vitreal anti-Leptospira antibody titers increase the probability of successful leptospiral culture from that sample [13,39,47]. If, as suspected, the dense round structures seen in the images by Brandes et al. [47] are biofilm, it seems possible that these structures may not be completely eliminated by macrophages.
Taking into account that the vitreous material as a heterogeneous viscous liquid cannot be equated with other tissues in every point, ERU fulfills all criteria described for biofilm-associated infections.
Neutrophil extracellular traps (NETs) have also been detected in vitreous samples from eyes affected by ERU [96]. Bacterial infections can initiate the formation of NETs, particularly when phagocytosis fails to eliminate the pathogen. Biofilms appear to be a particularly notable trigger for NET release. NETs, in turn, can stimulate biofilm formation [97,98]. Thus, a mutual positive feedback mechanism exists here, and mature, stable biofilms may be surrounded by larger amounts of NETs [98]. Although in the present study no NETs were visualized, this warrants further assessment in future studies.
Boundary tissues or “locus minoris resistentiae” are often located within avascular tissues of eye, kidney, joint, heart valves, arteries, and skin where there are reduced or absent inflammatory responses. As a result, pathogens can colonize and often persist at these sites [99]. It is interesting to consider the vitreous as a potential locus minoris resistentiae, with the capillaries of the ciliary body acting as the most likely site of entry into the vitreous cavity for Leptospira spp. As such, the vitreous body may be thought of as representing a 28 mL avascular immunological niche [13,39].
5. Conclusions
The clinical signs and chronic disease course of ERU, as well culturable Leptospira spp. in vitreous samples from these eyes, despite high vitreal antibody titers, apparent immune evasion, and ineffectiveness of antibiotics, fulfill all the criteria of a biofilm-associated infection. The previously known steps of biofilm formation have hereby been demonstrated in vitreous samples from equine eyes suffering from recurrent uveitis using both Warthin-Starry silver stain and immunohistochemistry. Thus, we conclude that ERU is a spontaneous disease due to in vivo biofilm formation by Leptospira spp. Future studies should further differentiate biofilm formation in the equine vitreous, analyze the composition of this biofilm, and provide insights for other biofilm-associated infections.
Acknowledgments
This work is dedicated to Siegfried Brem, who was always convinced that the intraocular anti-Leptospira antibodies indicated that the bacteria themselves must also be present in the eye. Without him, the successful Leptospira cultures with vitreous samples and all subsequent work over the past 25 years would have been impossible. Furthermore, we sincerely thank Maria Hauser from the State Office for Health and Food Safety, 85764 Oberschleissheim, Germany, for her always granted constructive helpfulness. Special thanks to David Maggs and Ron Settles for valuable suggestions and careful language revision.
Author Contributions
H.G. established vitrectomy in equine medicine and thus provided numerous ERU patients; H.G. and B.W. performed vitrectomies and thus provided the vitreous samples; B.W. initiated this study; K.A. performed ophthalmological examinations and vitreous sample preparation; K.A., R.K. and M.S. performed the staining procedures and microscopic examinations; H.G. bought the antibody; J.M. provided all other resources for the histological examinations; K.A. drafted the pictures; B.W., K.A. and R.K. wrote the manuscript; H.G. performed the critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
(Chair of Animal Welfare, Ethology, Animal Hygiene, and Animal Husbandry) Ethical review and approval were waived for this study because all samples used had been taken during therapeutic indicated vitrectomy and were waste of that surgery. No invasive procedure was required for the present study.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Høiby N., Bjarnsholt T., Moser C., Bassi G.L., Coenye T., Donelli G., Hall-Stoodley L., Holá V., Imbert C., Kirketerp-Møller K., et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015;21((Suppl. 1)):S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Jamal M., Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Hussain T., Ali M., Rafiq M., Kamil M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Goarant C., Trueba G., Bierque E., Thibeaux R., Davis B., de la Pena-Moctezuma A. Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project) UNESCO, Michigan State University; East Lansing, MI, USA: 2019. Part 3: Specific Excreted Pathogens: Environmental and Epidemiology Aspects-Section 2: Bacteria. [DOI] [Google Scholar]
- 5.Ristow P., Bourhy P., Kerneis S., Schmitt C., Prevost M.-C., Lilenbaum W., Picardeau M. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology. 2008;154:1309–1317. doi: 10.1099/mic.0.2007/014746-0. [DOI] [PubMed] [Google Scholar]
- 6.Thibeaux R., Soupé-Gilbert M.E., Kainiu M., Girault D., Bierque E., Fernandes J., Bähre H., Douyère A., Eskenazi N., Vinh J., et al. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. NPJ Biofilms Microbiomes. 2020;6:24. doi: 10.1038/s41522-020-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brihuega B., Samartino L., Auteri C., Venzano A., Caimi K. In vivo cell aggregations of a recent swine biofilm-forming isolate of Leptospira interrogans strain from Argentina. Rev. Argent Microbiol. 2012;44:138–143. [PubMed] [Google Scholar]
- 8.Yamaguchi T., Higa N., Okura N., Matsumoto A., Hermawan I., Yamashiro T., Suzuki T., Toma C. Characterizing interactions of Leptospira interrogans with proximal renal tubule epithelial cells. BMC Microbiol. 2018;18:64. doi: 10.1186/s12866-018-1206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer A.E., Crockett R., Kalsow C.M. Association of leptospiral seroreactivity and breed with uveitis and blindness in horses: 372 cases (1986-1993) J. Am. Vet. Med. Assoc. 1995;207:1327–1331. [PubMed] [Google Scholar]
- 10.Gerhards H., Wollanke B., Brem S. Vitrectomy as a diagnostic and therapeutic approach for equine recurrent uveitis (ERU); Proceedings of the 45th Annual Convention AAEP; Albuquerque, NM, USA. 8 December 1999; pp. 89–93. [Google Scholar]
- 11.Witmer R. Periodic ophthalmia in horses. Am. J. Ophthalmol. 1954;37:243–253. doi: 10.1016/0002-9394(54)91570-6. [DOI] [PubMed] [Google Scholar]
- 12.Crowhurst R.C. Periodic Ophthalmia: Clinical Aspects. Proc. R. Soc. Med. 1954;47:236–237. [PubMed] [Google Scholar]
- 13.Wollanke B. Habilitation Thesis. Ludwig-Maximilians-University; Munich, Germany: 2002. Die Equine Rezidivierende Uveitis (ERU) Als Intraokulare Leptospirose. [Google Scholar]
- 14.Komar G., Szutter L. Tieraerztliche Augenheilkunde. Paul Parey; Berlin, Germany: 1968. Die innere periodische Augenentzündung (Mondblindheit) der Pferde (Iridocyclitis recidiva equorum) pp. 231–237. [Google Scholar]
- 15.Kulbrock M., Von Borstel M., Rohn K., Distl O., Ohnesorge B. Studie zu Häufigkeit und Schweregrad der equinen rezidivierenden Uveitis bei Warmblütern. Pferdeheilkunde. 2013;29:27–36. doi: 10.21836/PEM20130105. [DOI] [Google Scholar]
- 16.Szemes P.A., Gerhards H. Untersuchungen zur Prävalenz der equinen rezidivierenden Uveitis im Großraum Köln-Bonn. Prakt. Tierarzt. 2000;81:408–420. [Google Scholar]
- 17.Gerding J.C., Gilger B.C. Prognosis and impact of equine recurrent uveitis. Equine Vet. J. 2016;48:290–298. doi: 10.1111/evj.12451. [DOI] [PubMed] [Google Scholar]
- 18.Gerhards H., Wollanke B. Surgical treatment of equine recurrent uveitis: Trans-pars-plana vitrectomy in horses. In: Gilger B.C., editor. Equine Ophthalmology. 1st ed. Elsevier Saunders; Philadelphia, PA, USA: 2005. pp. 314–319. [Google Scholar]
- 19.Werry H., Gerhards H. Technique and indications for surgical treatment of equine recurrent uveitis. Pferdeheilkunde. 1991;7:321. doi: 10.21836/PEM19910602. [DOI] [Google Scholar]
- 20.Werry H., Gerhards H. Surgical treatment of equine recurrent uveitis: A preliminary report. Tierarztl. Prax. 1992;20:178–186. [PubMed] [Google Scholar]
- 21.Wollanke B., Gerhards H., Schinagl C. Results of 654 trans-pars plana vitrectomies of equine eyes with recurrent uveitis-follow-up until 18 years after surgery. Pferdeheilkunde-Equine Med. 2021;37:204–214. doi: 10.21836/PEM20210301. [DOI] [Google Scholar]
- 22.Baake E.I.A., von Borstel M., Rohn K., Boevé M.H., Ohnesorge B. Long-term ophthalmologic examinations of eyes with equine recurrent uveitis after pars plana vitrectomy. Pferdeheilkunde. 2019;35:220–233. doi: 10.21836/PEM20190303. [DOI] [Google Scholar]
- 23.Von Borstel M., Von Oppen T., Glitz F., Frühauf B., Deegen E., Boevé M.H., Ohnesorge B. Long-term results of pars-plana (double-port) vitrectomy in equine recurrent uveitis. Pferdeheilkunde. 2005;21:13–18. doi: 10.21836/PEM20050102. [DOI] [Google Scholar]
- 24.Winterberg A., Gerhards H. Longterm-results of pars-plana-vitrectomy in equine recurrent uveitis. Pferdeheilkunde. 1997;13:377–383. doi: 10.21836/PEM19970409. [DOI] [Google Scholar]
- 25.Baumgart A., Gerhards H. Besonderheiten der Tigerschecken-Uveitis und möglicher Cyclosporin A-Einsatz in deren Therapie in Deutschland. Pferdeheilkunde. 2014;30:626–632. [Google Scholar]
- 26.Bryans J.T. Studies on equine leptospirosis. Cornell Vet. 1955;45:16–50. [PubMed] [Google Scholar]
- 27.Gsell O., Rehsteiner K., Verrey F. Iridocyclitis as a late consequence of Leptospirosis Pomona (porter’s disease): Agglutinin and lymphocytosis in the aqueous humor. Ophthalmologica. 1946;112:320–334. doi: 10.1159/000300399. [DOI] [PubMed] [Google Scholar]
- 28.Hartwigk H., Stoebbe E. Kultureller Nachweis von Leptospiren bei Hund und Pferd. Berl. Münch. Tierärztl. Wschr. 1952;65:188–190. [Google Scholar]
- 29.Morter R., Williams R., Bolte H., Freeman M.J. Equine leptospirosis. J. Am. Vet. Med. Assoc. 1969;155:436–442. [PubMed] [Google Scholar]
- 30.Roberts S.J. Sequelae of leptospirosis in horses on a small farm. J. Am. Vet. Med. Assoc. 1958;133:189–194. [PubMed] [Google Scholar]
- 31.Williams R.D. Master of Science Thesis. Purdue University; West Lafayette, IN, USA: 1968. The Presence and Duration of Persistence of Leptospira Pomona in Equine Ocular Tissues Following Experimentally Induced Systemic Infection. [Google Scholar]
- 32.Baake E., von Borstel M., Rohn K., Ohnesorge B. Detection of intraocular leptospiral DNA, antibodies and Leptospira spp. in horses with equine recurrent uveitis in different laboratories. Pferdeheilkunde. 2016;32:346–356. doi: 10.21836/PEM20160407. [DOI] [Google Scholar]
- 33.Borstel M.V., Oey L., Strutzberg-Minder K., Boevé M.H., Ohnesorge B. Direct and indirect detection of leptospires in vitreal samples of horses with ERU. Pferdeheilkunde. 2010;26:219–225. doi: 10.21836/PEM20100217. [DOI] [Google Scholar]
- 34.Dorrego Keiter E., Tóth J., Dikker L., Sielhorst J., Schusser G.F. Long-term results of pars plana vitrectomy in relationship to leptospiral antibody detection in vitreous humor in 118 horses with equine recurrent uveitis (ERU) Pferdeheilkunde-Equine Med. 2017;33:112–118. doi: 10.21836/PEM20170201. [DOI] [Google Scholar]
- 35.Dorrego-Keiter E., Tóth J., Dikker L., Sielhorst J., Schusser G.F. Detection of leptospira by culture of vitreous humor and detection of antibodies against leptospira in vitreous humor and serum of 225 horses with equine recurrent uveitis. Berl. Munch. Tierarztl. Wochenschr. 2016;129:209–215. [PubMed] [Google Scholar]
- 36.Toemoerdy E., Haessig M., Spiess B.M. The outcome of pars plana vitrectomy in horses with equine recurrent uveitis with regard to the presence or absence of intravitreal antibodies against various serovars of Leptospira interrogans. Pferdeheilkunde. 2010;26:251–254. doi: 10.21836/PEM20100222. [DOI] [Google Scholar]
- 37.Voelter K., Vial Z., Pot A.S., Spiess B.M. Leptospiral antibody prevalence and surgical treatment outcome in horses with Equine Recurrent Uveitis (ERU) in Switzerland. Vet. Ophthalmol. 2020;23:648–658. doi: 10.1111/vop.12767. [DOI] [PubMed] [Google Scholar]
- 38.Wollanke B., Gerhards H., Brem S., Kopp H., Meyer P. Intraocular and serum antibody titers to Leptospira in 150 horses with equine recurrent uveitis (ERU) subjected to vitrectomy. Berl. Munch. Tierarztl. Wochenschr. 1998;111:134–139. [PubMed] [Google Scholar]
- 39.Wollanke B., Gerhards H., Brem S., Meyer P., Kopp H. Ätiologie der equinen rezidivierenden Uveitis (ERU): Autoimmunkrankheit oder intraokulare Leptospireninfektion. Pferdeheilkunde. 2004;20:327–340. doi: 10.21836/PEM20040403. [DOI] [Google Scholar]
- 40.Wollanke B., Gerhards H., Brem S., Wolf E., Kopp H., Meyer P. Zur Leptospirenätiologie der equinen rezidivierenden Uveitis (ERU): Ergebnisse der Untersuchungen von Serum-und Glaskörperproben. Tieraerztl. Prax. 2000;28:153–158. [Google Scholar]
- 41.Wollanke B., Rohrbach B.W., Gerhards H. Serum and vitreous humor antibody titers in and isolation of Leptospira interrogans from horses with recurrent uveitis. J. Am. Vet. Med. Assoc. 2001;219:795–800. doi: 10.2460/javma.2001.219.795. [DOI] [PubMed] [Google Scholar]
- 42.Loibl J.K., Gerhards H., Brem S., Wollanke B. Improving the laboratory diagnosis of leptospiral uveitis in horses by using an indirect ELISA for the detection of antibodies against Leptospira spp. in intraocular samples. Pferdeheilkunde. 2018;34:267–277. doi: 10.21836/PEM20180308. [DOI] [Google Scholar]
- 43.Wollanke B., Geiger T., Gerhards H. Evaluation of" SNAP (R) Lepto"-ELISA and comparison with MAT and PCR results for diagnosis of leptospiral uveitis in horses using intraocular samples. Pferdeheilkunde. 2018;34:508–516. doi: 10.21836/PEM20180601. [DOI] [Google Scholar]
- 44.Faber N.A., Crawford M., LeFebvre R.B., Buyukmihci N.C., Madigan J.E., Willits N.H. Detection of Leptospira spp. in the aqueous humor of horses with naturally acquired recurrent uveitis. J. Clin. Microbiol. 2000;38:2731–2733. doi: 10.1128/JCM.38.7.2731-2733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polle F., Storey E., Eades S., Alt D., Hornsby R., Zuerner R., Carter R. Role of intraocular Leptospira infections in the pathogenesis of equine recurrent uveitis in the southern United States. J. Equine Vet. Sci. 2014;34:1300–1306. doi: 10.1016/j.jevs.2014.09.010. [DOI] [Google Scholar]
- 46.Popp M., Cerhards H., Wollanke B. Enrofloxacin concentrations in the vitreous of horses with equine recurrent uveitis (ERU) after repeated intravenous administration. Pferdeheilkunde. 2013;29:574–580. doi: 10.21836/PEM20130501. [DOI] [Google Scholar]
- 47.Brandes K., Wollanke B., Niedermaier G., Brem S., Gerhards H. Recurrent uveitis in horses: Vitreal examinations with ultrastructural detection of leptospires. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007;54:270–275. doi: 10.1111/j.1439-0442.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 48.Niedermaier G., Wollanke B., Hoffmann R., Brem S., Gerhards H. Detection of leptospira in the vitreous body of horses without ocular diseases and of horses with equine recurrent uveitis (ERU) using transmission-electron microscopy. Dtsch. Tierarztl. Wschr. 2006;113:401–432. [PubMed] [Google Scholar]
- 49.Degroote R.L., Deeg C.A. Immunological Insights in Equine Recurrent Uveitis. Front. Immunol. 2021;11:609855. doi: 10.3389/fimmu.2020.609855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeg C.A. Ocular immunology in equine recurrent uveitis. Vet. Ophthalmol. 2008;11((Suppl. 1)):61–65. doi: 10.1111/j.1463-5224.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 51.Voigt V., Wikstrom M.E., Kezic J.M., Schuster I.S., Fleming P., Makinen K., Daley S.R., Andoniou C.E., Degli-Esposti M.A., Forrester J.V. Ocular antigen does not cause disease unless presented in the context of inflammation. Sci. Rep. 2017;7:14226. doi: 10.1038/s41598-017-14618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavach J.D. Vitreous. In: Stamathis G., editor. Large Animal Ophthalmology. Mosby Company; St Louis, MO, USA: 1990. pp. 202–205. [Google Scholar]
- 53.Niedermaier G., Wollanke B., Hoffmann R., Matiasek K., Gerhards H. Darstellung der Glaskörperstruktur von augengesunden Pferden und von Pferden mit equiner rezidivierender Uveitis (ERU) mittels Transmissions-Elektronenmikroskopie. Dtsch. Tierarztl. Wschr. 2006;113:209–248. [PubMed] [Google Scholar]
- 54.Sebag J. The Vitreous: Structure, Function and Pathobiology. 1st ed. Springer; New York, NY, USA: 1989. [Google Scholar]
- 55.Geißler P., Wollanke B. Biofilm formation in persistent infections and its role in the pathogenesis of equine recurrent uveitis (ERU)-a literature review. Pferdeheilkunde-Equine Med. 2021;37:225–233. doi: 10.21836/PEM20210303. [DOI] [Google Scholar]
- 56.Riedelsheimer B., Buechl-Zimmermann S. Faerbungen. In: Mulisch M., Welsch U., editors. Romeis Mikroskopische Technik. 19th ed. Springer; Berlin/Heidelberg, Germany: 2015. pp. 234–235. [Google Scholar]
- 57.Sapi E., Bastian S.L., Mpoy C.M., Scott S., Rattelle A., Pabbati N., Poruri A., Burugu D., Theophilus P.A.S., Pham T.V., et al. Characterization of biofilm formation by Borrelia burgdorferi In Vitro. PLoS ONE. 2012;7:e48277. doi: 10.1371/journal.pone.0048277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapi E., Balasubramanian K., Poruri A., Maghsoudlou J.S., Socarras K.M., Timmaraju A.V., Filush K.R., Gupta K., Shaikh S., Theophilus P.A., et al. Evidence of In Vivo Existence of Borrelia Biofilm in Borrelial Lymphocytomas. Eur. J. Microbiol. Immunol. 2016;6:9–24. doi: 10.1556/1886.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Domenico E.G., Cavallo I., Bordignon V., D′Agosto G., Pontone M., Trento E., Gallo M.T., Prignano G., Pimpinelli F., Toma L., et al. The Emerging Role of Microbial Biofilm in Lyme Neuroborreliosis. Front. Neurol. 2018;9:1048. doi: 10.3389/fneur.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monahan A.M., Callanan J.J., Nally J.E. Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet. Pathol. 2009;46:792–799. doi: 10.1354/vp.08-VP-0265-N-REV. [DOI] [PubMed] [Google Scholar]
- 61.Bjarnsholt T., Jensen P., Fiandaca M.J., Pedersen J., Hansen C.R., Andersen C.B., Pressler T., Givskov M., Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 62.Hall-Stoodley L., Stoodley P., Kathju S., Høiby N., Moser C., Costerton W.J., Moter A., Bjarnsholt T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012;65:127–145. doi: 10.1111/j.1574-695X.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 63.Høiby N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014;70:205–211. doi: 10.1111/2049-632X.12165. [DOI] [PubMed] [Google Scholar]
- 64.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 65.Dowsett C. Biofilms: A practice-based approach to identification and treatment. Wounds UK. 2013;9:68–72. [Google Scholar]
- 66.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 67.Coffin D.L. Detection of leptospires by fluorescent antibody. Am. J. Vet. Res. 1962;23:159–164. [PubMed] [Google Scholar]
- 68.Cole J.R., Jr., Sulzer C.R., Pursell A.R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl. Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribotta M.J., Higgins R., Gottschalk M., Lallier R. Development of an indirect enzyme-linked immunosorbent assay for the detection of leptospiral antibodies in dogs. Can. J. Vet. Res. 2000;64:32–37. [PMC free article] [PubMed] [Google Scholar]
- 70.Maruoka T., Nikaido Y., Miyahara S., Katafuchi E., Inamasu Y., Ogawa M., Fukuda K., Nakayama T., Horishita T., Saito M. Correlation between renal distribution of leptospires during the acute phase and chronic renal dysfunction in a hamster model of infection with Leptospira interrogans. PLoS Negl. Trop. Dis. 2021;15:e0009410. doi: 10.1371/journal.pntd.0009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blenden D.C., Goldberg H.S. Silver impregnation stain for Leptospira and Flagella. J. Bacteriol. 1965;89:899–900. doi: 10.1128/jb.89.3.899-900.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Churukian C.J., Schenk E.A. A Warthin-Starry method for spirochetes and bacteria using a microwave oven. J. Histotechnol. 1988;11:149–151. doi: 10.1179/his.1988.11.3.149. [DOI] [Google Scholar]
- 73.Thomas C. Histopathologie Kompakt: Kursbuch der Allgemeinen und Speziellen Histopathologie. 1st ed. Schattauer Verlag; Stuttgart, Germany: 2004. [Google Scholar]
- 74.Almeida D.S., Paz L.N. Investigation of chronic infection by Leptospira spp. in asymptomatic sheep slaughtered in slaughterhouse. PLoS ONE. 2019;14:e0217391. doi: 10.1371/journal.pone.0217391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saglam Y., Yener Z. Immunohistochemical detection of leptospiral antigens in cases of naturally occurring abortions in sheep. Small Rumin. Res. 2008;74:119–122. doi: 10.1016/j.smallrumres.2007.04.006. [DOI] [Google Scholar]
- 76.Santos A.A., Figueira C.P. Heterogenic colonization patterns by Leptospira interrogans in Rattus norvegicus from urban slums. Braz. J. Microbiol. 2015;46:1161–1164. doi: 10.1590/S1517-838246420140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutherland I.W. The biofilm matrix--an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9:222–227. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 78.Garrett T.R., Bhakoo M., Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008;18:1049–1056. doi: 10.1016/j.pnsc.2008.04.001. [DOI] [Google Scholar]
- 79.Maric S., Vranes J. Characteristics and significance of microbial biofilm formation. Period. Biol. 2007;109:115–121. [Google Scholar]
- 80.Wild C.J., Greenlee J.J., Bolin C.A., Barnett J.K., Haake D.A., Cheville N.E. An improved immunohistochemical diagnostic technique for canine leptospirosis using antileptospiral antibodies on renal tissue. J. Vet. Diagn. Investig. 2002;14:20–24. doi: 10.1177/104063870201400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zilber A.L., Belli P., Artois M., Kodjo A., Djelouadji Z. First Observation of Leptospira interrogans in the Lungs of Rattus norvegicus. Biomed. Res. Int. 2016;2016:9656274. doi: 10.1155/2016/9656274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parsek M.R., Singh P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 83.Hall-Stoodley L., Stoodley P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 84.Linke R.P., Brandes K., Cielewicz M.-B., Gerhards H., Wollanke B. Ocular leptospiral infection leads to ciliary induction and local AA-amyloidosis in horses. Amyloid. 2019;26:127–128. doi: 10.1080/13506129.2019.1584100. [DOI] [PubMed] [Google Scholar]
- 85.Waldner J., Gerhards H., Wollanke B. Investigations into the occurrence of serum amyloid A in the equine eye. Pferdeheilkunde–Equine Med. 2018;34:461–467. doi: 10.21836/PEM20180501. [DOI] [Google Scholar]
- 86.Vinod Kumar K., Lall C., Vimal Raj R., Vedhagiri K., Vijayachari P. Molecular detection of pathogenic leptospiral protein encoding gene (lipL32) in environmental aquatic biofilms. Lett. Appl. Microbiol. 2016;62:311–315. doi: 10.1111/lam.12533. [DOI] [PubMed] [Google Scholar]
- 87.Brem S., Gerhards H., Wollanke B., Meyer P., Kopp H. Demonstration of intraocular leptospira in 4 horses suffering from equine recurrent uveitis (ERU) Berl. Munch. Tierarztl. Wochenschr. 1998;111:415–417. [PubMed] [Google Scholar]
- 88.Brem S. (State Office for Health and Food Safety, 85764 Oberschleißheim, Germany) Personal Communication. 1996.
- 89.Bohl E., Ferguson L. Leptospirosis in domestic animals. J. Am. Vet. Med. Assoc. 1952;121:421–428. [PubMed] [Google Scholar]
- 90.Halliwell R.E., Brim T.A., Hines M.A., Wolf D., White F.H. Studies on equine recurrent uveitis. II: The role of infection with Leptospira interrogans serovar pomona. Curr. Eye Res. 1985;4:1033–1040. doi: 10.3109/02713688509003348. [DOI] [PubMed] [Google Scholar]
- 91.Hines M.T. Immunologically mediated ocular disease in the horse. Vet. Clin. North. Am. Large Anim. Pract. 1984;6:264–271. doi: 10.1016/S0196-9846(17)30006-X. [DOI] [PubMed] [Google Scholar]
- 92.Williams R.D., Morter R.L., Freeman M.J., Lavignette A.M. Experimental chronic uveitis. Ophthalmic signs following equine leptospirosis. Investig. Ophthalmol. 1971;10:948–954. [PubMed] [Google Scholar]
- 93.Dubielzig R., Render J., Morreale R. Distinctive morphologic features of the ciliary body in equine recurrent uveitis. Vet. Comp. Ophthalmol. 1997;7:163–167. [Google Scholar]
- 94.Romeike A., Brügmann M., Drommer W. Immunohistochemical studies in equine recurrent uveitis (ERU) Vet. Pathol. 1998;35:515–526. doi: 10.1177/030098589803500606. [DOI] [PubMed] [Google Scholar]
- 95.Roth T., Brandes K., Gerhards H., Giving E., Wollanke B. Histologische Untersuchungen des Glaskörpers bei Pferden mit equiner rezidivierender Uveitis. Pferdeheilkunde. 2014;30:512–520. doi: 10.21836/PEM20140501. [DOI] [Google Scholar]
- 96.Fingerhut L., Ohnesorge B., von Borstel M., Schumski A., Strutzberg-Minder K., Mörgelin M., Deeg C.A., Haagsman H.P., Beineke A., von Köckritz-Blickwede M., et al. Neutrophil Extracellular Traps in the Pathogenesis of Equine Recurrent Uveitis (ERU) Cells. 2019;8:1528. doi: 10.3390/cells8121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 98.Papayannopoulos V. Neutrophils Facing Biofilms: The Battle of the Barriers. Cell Host Microbe. 2019;25:477–479. doi: 10.1016/j.chom.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 99.Schulz L.-C., Hermanns W. Zur Bedeutung der Grenzflächengewebe bei der rheumatoiden Entzündung. In: Deicher H., editor. Pathomechanismen Entzündlicher Rheumatischer Erkrankungen Bei Mensch und Tier. DFG, Sonderforschungsbereiche; Berlin, Germany: 1989. pp. 327–341. [Google Scholar]