Table 3.
Chemical structures of synthesized oxime derivatives and JNK binding affinity.
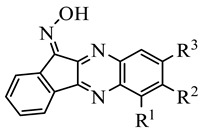
| Compound | R1 | R2 | R3 | JNK1 | JNK2 | JNK3 |
|---|---|---|---|---|---|---|
| Kd (µM) | ||||||
| IQ-1 * | H | H | H | 0.24 | 0.36 | 0.10 |
| 4a | H | F | H | 0.28 ± 0.07 | 0.62 ± 0.01 | 0.19 ± 0.01 |
| 4b | Cl | H | H | 0.17 ± 0.04 | 0.22 ± 0.06 | 0.14 ± 0.03 |
| 4c | CH3 | H | Br | N.B. | N.B. | N.B. |
| 4d | H | t-Bu | H | 3.1 ± 0.1 | 1.6 ± 0.6 | 3.1 ± 0.2 |
| 4e | F | H | F | 0.92 ± 0.05 | 1.6 ± 0.01 | 0.9 ± 0.3 |
| 4f | H | F | F | 0.17 ± 0.04 | N.B. | 0.52 ± 0.3 |
| 4g | F | F | H | 0.52 ± 0.09 | 0.91 ± 0.06 | 1.1 ± 0.1 |
| 4h | Br | H | CF3 | N.B. | N.B. | N.B. |
| 4i | Cl | H | CF3 | N.B. | N.B. | N.B. |
| 4j | CF3 | H | CF3 | N.B. | N.B. | N.B. |
| 4k | H | F | Cl | N.B. | N.B. | 24.5 ± 2.1 |
| 4l | H | H | COOCH3 | 0.91 ± 0.16 | 2.4 ± 0.2 | 0.71 ± 0.05 |
| 4m | CH2MRF | H | H | 1.1 ± 0.1 | 0.88 ± 0.10 | 0.91 ± 0.3 |
* Data for IQ-1 are from [35]. N.B., no binding affinity at concentrations < 30 μM. Abbreviations: MRF, morpholine.
