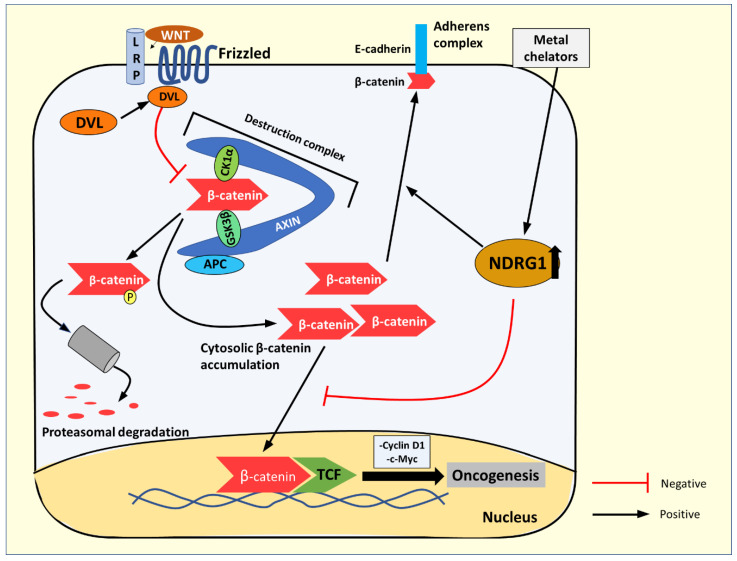
Figure 10.
The Wnt/β-catenin signaling pathway and NDRG1’s effect on β-catenin localization. In the absence of the Wnt ligand, β-catenin is phosphorylated by the destruction complex (consisting of casein kinase 1α (CK1α), glycogen synthase kinase 3β (GSK3β), the tumor suppressor APC, scaffold protein AXIN, and others (not shown)) leading to its proteasomal degradation. The Wnt ligand binds to and activates the LRP5/6 and Frizzled co-receptors. The activated receptor then recruits Dishevelled (DVL), where it activates and then sequesters the “destruction complex”, which inhibits its activity, allowing non-phosphorylated β-catenin to accumulate in the cytosol and then translocate to the nucleus. Here β-catenin acts as a co-activator with the T cell factor (TCF) family of transcription factors, which up-regulate proteins such as cyclin D1 and c-myc that promote oncogenesis. NDRG1 up-regulation by iron-binding ligands such as DFO or Dp44mT inhibits β-catenin from translocating to the nucleus, preventing its transactivation activity and promoting cytosolic β-catenin localization to the membrane to form part of the adherens complex.