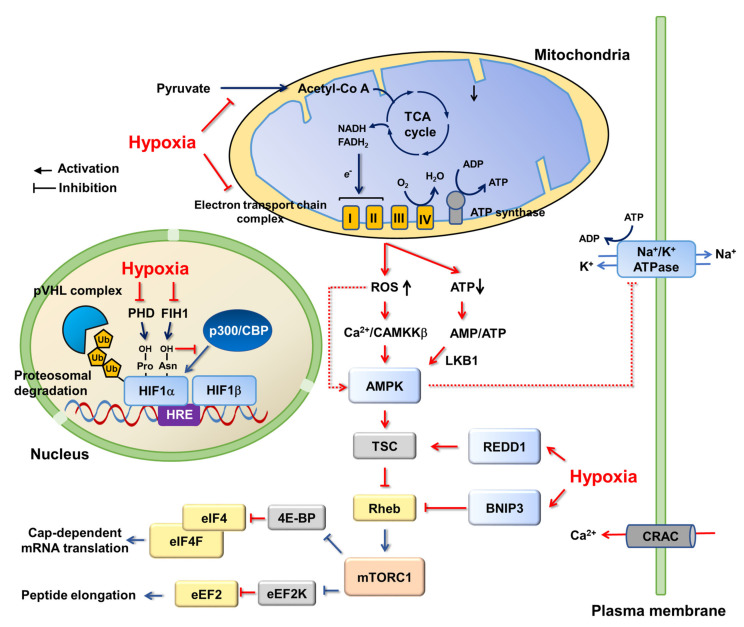
Figure 3.
AMPK, mTORC1, and HIF1 in hypoxia. Hypoxia inhibits the mitochondrial electron transport chain complex to impair ATP synthesis. It can cause increases in intracellular ROS level, which increases intracellular Ca2+ to activate AMPK, independent of any AMP/ATP change. When hypoxia lasts hours, intracellular AMP/ATP level is increased, which further enhances or maintains AMPK activation. AMPK inhibits mTORC1 via TSC, which is also directly activated by hypoxia through REDD1. In addition, hypoxia-induced BNIP3 disrupts Rheb-mTORC1 interaction, thereby leading to mTORC1 inhibition. Inactivation of mTORC1 in hypoxia causes the deceleration of protein translation at both initiation (by inhibiting eIF4F mRNA cap-binding complex) and elongation (by activating eEF2 kinase, a negative regulator of elongation factor eEF2) steps. Additionally, hypoxia stabilizes a heterodimeric transcription factor HIF by inhibiting HIFα proline hydroxylase PHD, which prevents the interaction between HIFα and E3 ubiquitin ligase pVHL complex. Molecular pathways regulated by hypoxia are shown in red.