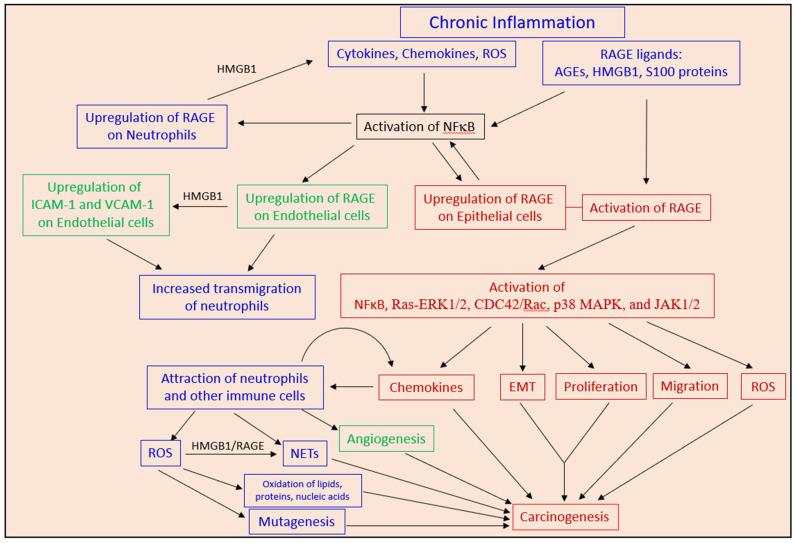
Figure 1.
Chronic inflammation-induced carcinogenesis. The figure illustrates pathways in chronic neutrophilic inflammation-induced carcinogenesis that involves the activation of the RAGE signaling pathways in neutrophils (blue boxes), endothelial cells (green boxes) and epithelial cells (brown boxes). The different circuits and pathways are discussed in more details in other parts of the review. Briefly, the chronic inflammation is characterized by neutrophil production of cytokines, chemokines and reactive oxygen species (ROS) that leads to a continuous activation of NFκB in neutrophils, endothelial and epithelial cells, resulting in the upregulation of RAGE, which, in turn, becomes activated by its many ligands present in the inflamed area. Among them, HMGB1, which is released upon tissue injury and NETosis, plays a particular role in activating endothelial cells and in inducing proliferation and migration of epithelial cells. HMGB1 also modulates neutrophil functions. The activated endothelial cells upregulate the adhesion molecules ICAM-1 and VCAM-1 that facilitate neutrophil endothelial transmigration. Activation of RAGE in epithelial cells leads to the production of chemokines that attract more neutrophils and other immune cells, thereby, aggravating the inflammatory process. Other RAGE ligands involved in inflammation-induced carcinogenesis include advanced glycation end products (AGEs) that are proteins or lipids that have become glycated after exposure to excess sugars and S100 proteins, such as S100A4 and S100A7 produced by tumor cells and S100A8/S100A9 produced by neutrophils. The prolonged exposure of the epithelial cells to RAGE ligands, NETs, chemokines, ROS and other stress stimuli, ultimately leads to the initiation of carcinogenesis.