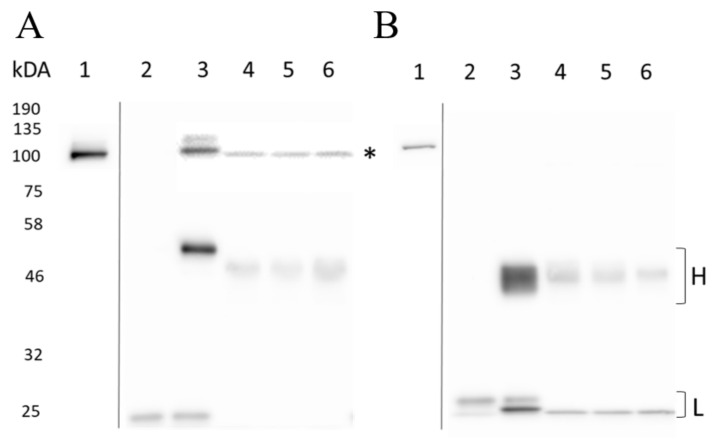
Figure 9.
Comparison of antibodies and Affimers for immunoprecipitation of EPAC1 and EPAC2 using. (A) EPAC1 and (B) EPAC2 U20S cells were harvested in 0.5 mL of RIPA buffer, containing protease inhibitor cocktail, and lysed for 30 min by rotation at 4 °C. Extracts were centrifuged and cell lysates were collected, including inputs (total EPAC protein) (lane 1). To each cell lysate, 4 µg of rabbit IgG antibody (lane 2), 1.4 µg of 5D3 (A: lane 3) or 2.5 µg of 5B1 antibody (B: lane 3), or 2.6 µg of Affimer: 780A (lane 4), 380A (lane 5) and 414A (lane 6) were added and incubated with rotation for one hour (4 °C) before adding 40 µL of Protein G Agarose (lanes 2–4) or α-His-Tag Sepharose® beads (lanes 5–6). After a one-hour incubation at 4 °C, with rotation, antibody-bead complexes were collected while Affimer-bead complexes were collected after an overnight incubation at 4 °C. Beads were then washed five times with 0.5 mL RIPA buffer using centrifugation for the collection of beads. Samples were heated in electrophoresis sample buffer for five minutes at 95 °C and analyzed by Western blotting where 15 and 30 µL of input and isolated immunoprecipitated proteins, respectively, per well from EPAC1 or EPAC2 U20S cells were separated by SDS-PAGE and probed with (A) 5D3 antibody or (B) 5B1 and α-mouse primary and secondary antibodies, respectively, as described in Materials and Methods. Total EPAC Protein (*), and heavy (H) and light (L) chain bands are indicated. Protein markers (kDa) are also indicated.