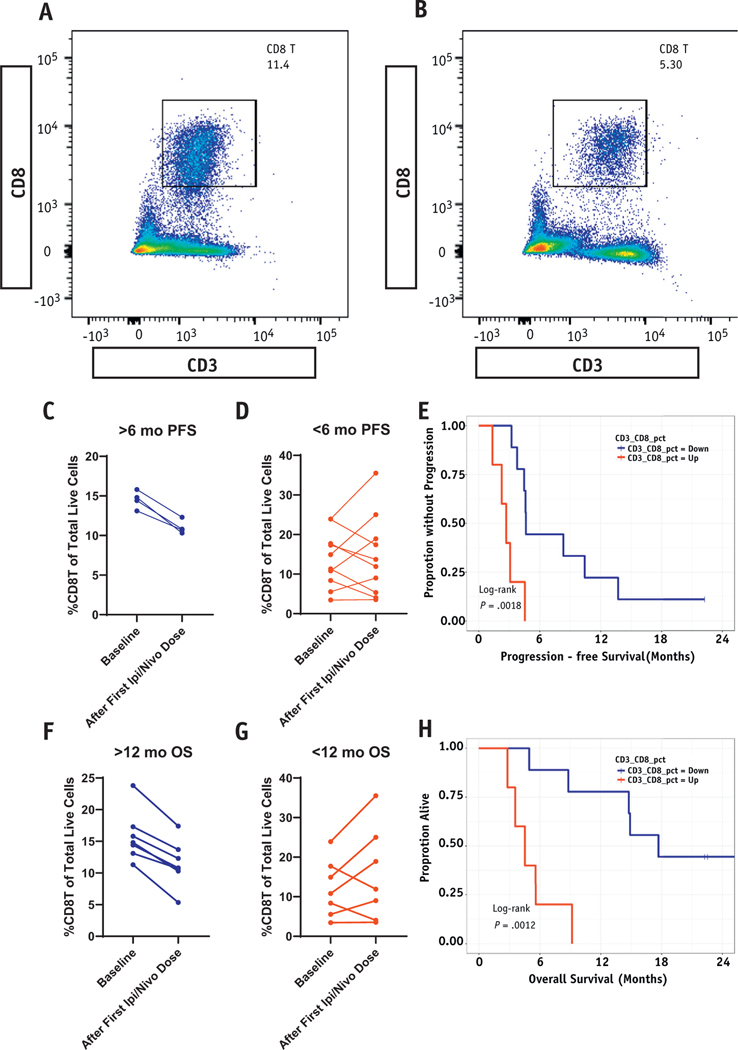
Fig. 5.
Reduction in peripheral CD3+CD8+ predicts for study primary and secondary endpoints. (A and B) Representative flow cytometry gating cell populations to demonstrate reduction in CD3+CD8+ from baseline to after treatment with ipilimumab/nivolumab. (C) %CD3+CD8+ at baseline and after first dose of ipilimumab and nivolumab among 4 patients achieving 6-month progression-free survival (PFS; primary endpoint). (D) %CD3+CD8+ among patients with PFS <6 months (n = 10). (E) Kaplan-Meier PFS (primary endpoint) based on whether CD3+CD8+ cells were increasing or decreasing after treatment with ipilimumab and nivolumab. (F) %CD3+CD8+ at baseline and after first dose of ipilimumab and nivolumab among 6 patients achieving 12-month overall survival (OS; secondary endpoint). (G) %CD3+CD8+ among patients with OS <12 months (n = 8). (H) Kaplan-Meier overall survival (secondary endpoint) based on whether CD3+CD8+ cells were increasing or decreasing after treatment with ipilimumab and nivolumab.