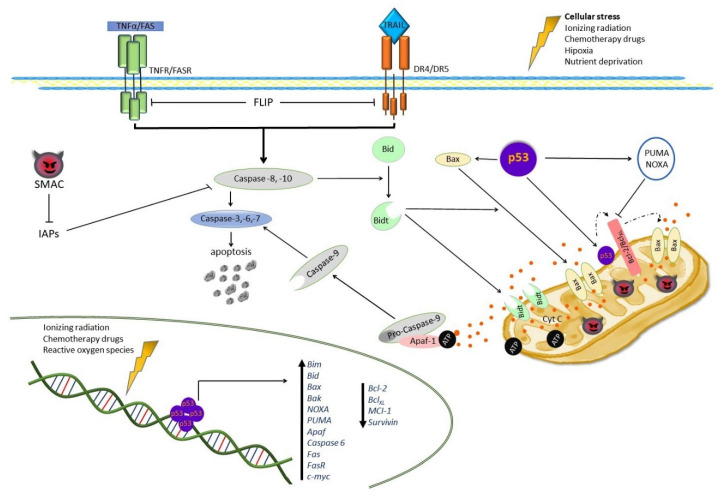
Figure 3.
Extrinsic and intrinsic apoptosis pathway and its regulation via p53. Under cellular stress, the extrinsic pathway is started by ligand binding to death receptors, including TNFα, Fas and TRAIL; this leads to the autoactivation of caspases-8 and -10, which in turn promote the catalytic activation of the effector caspase-3. Another target of caspase-8 is the pro-apoptotic protein Bid, which is hydrolyzed to tBid, inducing Bax oligomerization and mitochondria depolarization with release of cyt c. Along with the activation of caspase-9, these events amplify the apoptotic pathway. The intrinsic pathway involves the permeabilization of the mitochondrial external membrane, which facilitates the cytosolic release of pro-apoptotic proteins like SMAC/Diablo and cyt c, which are otherwise confined within the intermembrane space. cyt c binds the Apaf-1 protein, which in turn binds and activates caspase-9, responsible for the activation of apoptosis executioners: Caspases-3, -6, and -7. On the other hand, SMAC/Diablo inhibits IAPs, which bind and neutralize caspases-8 and -10. Another protein involved in apoptosis regulation is p53, which transcriptionally activates pro-apoptotic genes and inhibits anti-apoptotic genes, directly inhibiting BclXL and Bcl-2 in the mitochondria, favoring apoptosis. Continue arrows (↓) indicate activation, arrows with (⊥) indicate inhibition.