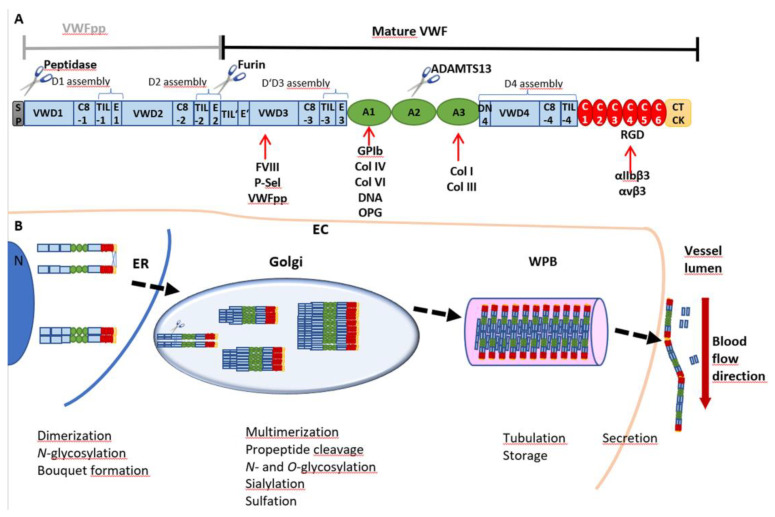
Figure 1.
VWF domain structure and multimer biosynthesis. (A) VWF is synthesized as a pre-pro-monomer with a signal peptide (SP) and a pro-peptide (VWFpp) containing the D1 and D2 assemblies, each consisting of a VWD, C8, TIL and E domain. The VWFpp is cleaved off by furin, leaving the mature VWF, including the D’D3 assembly and domains A1, A2 and A3, followed by the D4 assembly, C1-C6 and the CTCK domains. Cleavage and thus degradation of VWF can occur between Tyr1605 and Met1606 of the A2 domain by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Binding partners are indicated below the respective domains: coagulation factor VIII (FVIII), P-selectin (P-sel) and the VWFpp interact with D’D3; the A1 domain is the binding site for glycoprotein Ibα (GPIbα), collagens (Col) IV and VI, DNA and osteoprotegerin (OPG); collagens I and III bind to A3; and the RGD motif in C4 is the interaction site for integrins αIIbβ3 (= receptor GPIIb/IIIa) and αvβ3. (B) The SP guides translation into the endoplasmic reticulum (ER), where monomers are dimerized by the formation of three disulfide bonds between the CTCK domains. Further N-glycosylation is initiated, and dimers arrange into a “bouquet-like” formation with a closed stem region. In the Golgi apparatus, dimers are multimerized by disulfide bonds between D’D3 domains, and VWF is heavily glycosylated, sialylated and sulfated. The VWFpp is cleaved but stays non-covalently attached and supports multimerization and, later on, tubule formation, packaging and storage in Weibel–Palade bodies (WPB). After secretion into the vessel lumen, the VWFpp is released.