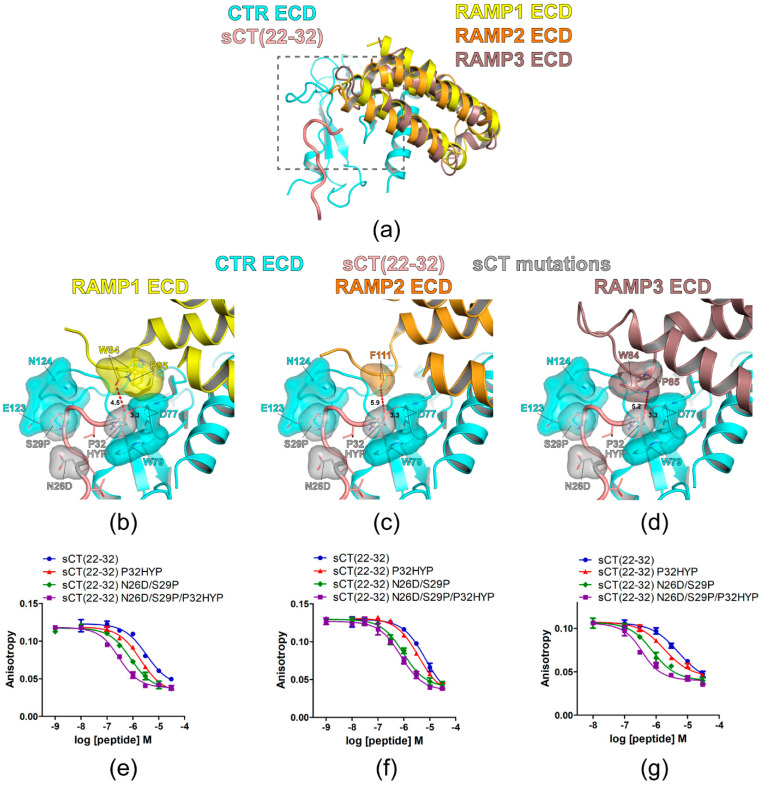
Figure 5.
Hypothetical structures of AMY receptor ECDs and the peptide binding assay with sCT(22–32) N26D/S29P/P32HYP mutations. (a) Overview of the hypothetical structures of AMY receptor 1/2/3 ECDs. The crystal structure of CTR ECD (PDB 6PFO, Mol A) with sCT(22–32) and RAMP 1/2/3 ECDs structures from the CGRP (PDB 6E3Y) and AM1/2 receptors (PDB 6UUN and 6UVA) were used to build hypothetical AMY receptor 1/2/3 ECDs. The peptide binding pocket and proximal RAMP ECD residues were boxed with a gray dotted line. (b–d) Hypothetical structures of AMY receptor 1/2/3 ECDs with sCT(22–32) N26D/S29P/P32HYP mutations. CTR ECD D77, W79, H121, E123, and N124, sCT N26D, S29P, and P32HYP mutated residues, and RAMP ECD residues (W84 and P85 in RAMP1/3 ECD and F111 in RAMP2 ECD) were shown both with stick and surface representations. The distances between sCT HYP32 and the proximal RAMP ECD residue and between sCT HYP32 and the CTR ECD D77 main chain were shown as a dotted red line. For (c), the side chain of F111 of RAMP2 ECD was unavailable in the original cryo-EM structure (PDB 6UUN) and only the first carbon of the side chain was shown as stick and surface representations. (e–g) FP competition peptide binding assay with sCT(22–32) N26D/S29P/P32HYP mutations. FITC-AC413(6–25) with Y25 mutation was used as a peptide probe. Representative peptide-binding curves were shown from three independent experiments.