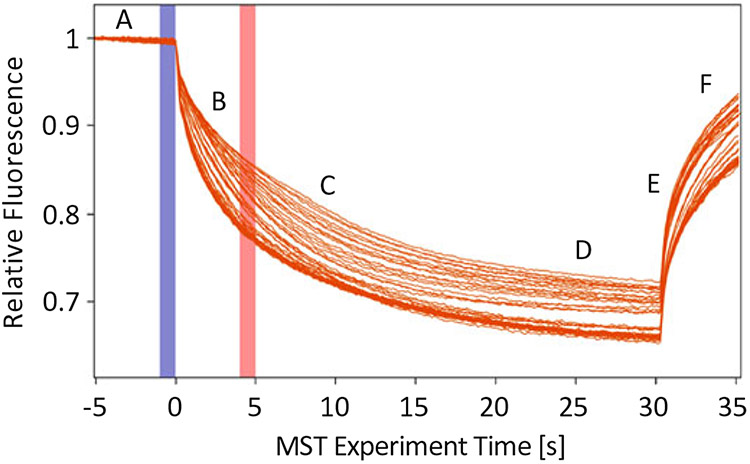
Fig. 1.
Thermophoretic movement of Sec18 monomer bound to PA nanodiscs labeled with Atto 647 dye. This is a sample output using M.O. Affinity Analysis software V2.1.5 of Sec18 Monomer titrated against PA nanodiscs as described in Subheading 3.2 exporting chart from the Dose Response Fit Screen and defined according to [1]. (A) Initial fluorescence: Measurement taken at room temperature prior to turning on IR laser. (B) T-Jump: Fluorescence measurement differences present when IR laser is first turned on prior to thermophoretic movement of particles being measured. (C) Thermophoresis: Fluorescence change due to thermophoretic motion differences due to factors such as size, charge, solvation entropy, or conformational change of particle being measured with respect to titrated analyte. (D) Steady state: Equilibrium achieved between all capillaries containing different concentrations of analyte, generally occurring between 10 and 30 s. (E) Inverse T-Jump: Fluorescence measurement differences present when IR laser is first turned off after thermophoretic equilibrium or near equilibrium. (F) Back diffusion: Returning of relative fluorescence back to initial, however, may not reach the same value due to photobleaching (n = 3)