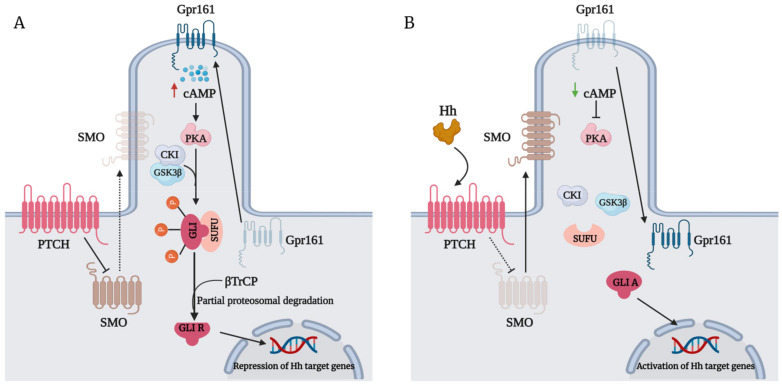
Figure 1.
(A) The repression of Smoothened (SMO) by the Patched (PTCH) receptor in the absence of hedgehog (Hh) ligands promotes the interaction of Suppressor of Fused (SUFU) and glioma-associated oncogene homolog (GLI). G-protein coupled receptor 61 (GPR161) translocates to the primary cilium, which triggers high levels of cyclic adenosine monophosphate (CAMP). Elevated ciliary levels of CAMP maintain high levels of protein kinase A (PKA) activity, which phosphorylate GLI at P1-6 clusters. Consequently, phosphorylation of GLI by PKA prime its phosphorylation by casein kinase I (CKI) and glycogen synthase kinase 3 beta (GSK3β) further. Phosphorylated GLI is recognized by the β-TrCP, promoting its ubiquitination and partial proteasomal processing into a repressor. GLI repressor (GLIR) then translocates into the nucleus to repress target gene transcription. (B) The binding of the Hh ligand to the PTCH receptor alleviates its repression of SMO, allowing SMO translocation to the primary cilium. Activated SMO inhibits SUFU, allowing the dissociation of GLI from SUFU. Additionally, Gpr161 is removed from the primary cilium, causing low CAMP levels and PKA activity. The release of GLI from SUFU and low PKA activity results in the dephosphorylation of GLI, preventing its proteasomal processing into a repressor. Full-length GLI or GLI activator (GLIA) then translocates into the nucleus to transcribe target genes. Red upward triangle-headed arrow: upregulation; green downward triangle-headed arrow: downregulation; dotted black triangle-headed arrow: inactivation; bar-headed arrow: inhibition; dotted bar-headed arrow: loss of inhibition.