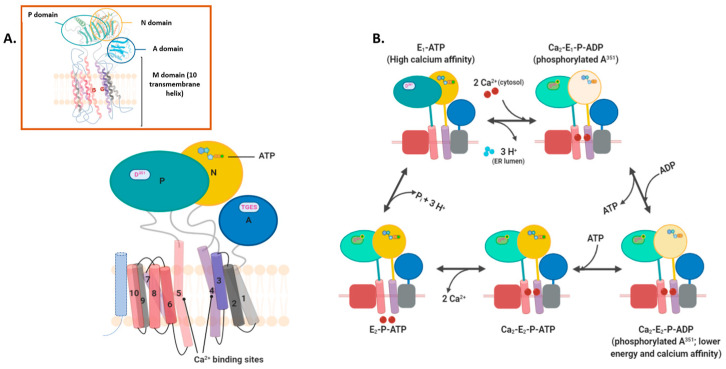
Figure 3.
Sarcoplasmic endoplasmic Ca2+-ATPase structure and mechanism of Ca2+ ion transport. (A) SERCA pump structure is comprised of Actuator or A domain (for dephosphorylation), transmembrane or M domain (10 helices and two Ca2+ ion binding sites between helices 4 and 5), Nucleotide or N domain (source of phosphorylation), and Phosphorylation or P domain (consists of the site of phosphorylation; aspartate 351 residue) [26]. Picture in the inset is a simplified representation of SERCA crystal structure; (B) Numerous steps are involved in the transport of Ca2+ via SERCA across the endoplasmic reticulum membrane [28,29,30,31]. As per the above model, the pump cycle begins with SERCA having a high affinity to Ca2+ in its physiologically predominant E1-ATP state. After cytosolic Ca2+ ions bind to SERCA, the pump transitions into Ca2-E1-P-ADP state utilizing the energy from the transfer of ATP γ-phosphate in the N domain to D351 amino acid residue in P domain. Transport of Ca2+ ions to the ER lumen parallels the shift of pump from its high-energy intermediate conformation to the low energy E2-ATP state (with less affinity to Ca2+; not shown) triggered by replacement of ADP with ATP. Ca2+ ions are then exchanged in the lumen with 2–3 protons compensating for the loss of positive charge. Followed by dephosphorylation at D351. In a Ca2+-free resting stage, the γ-phosphate of ATP in N-domain is masked from phosphorylation site (P domain Asp351) as well as the dephosphorylation site (A-domain TGES sequence) [25,32]. When two cytosolic Ca2+ ions bind to SERCA pump in its higher Ca2+ affinity state (known as E1-ATP), it results in rotational changes within the transmembrane and cytoplasmic domains, thus bringing N-domain ATP closer to Asp351 for transfer of γ-phosphate. From this high-energy intermediate state (Ca2-E1-P-ADP) the pump prepares to transition into a lower energy state, Ca2-E2-P-ADP by completely transferring the γ-phosphate to P-domain and coordinating Mg2+ ions to all the phosphate groups present. In a two-step process again, first ADP is exchanged for ATP in the ER causing appropriate conformational changes to create a channel and exposing the Ca2+-binding residues in its lumen (E2P-ATP). Ca2+ ions are then released in exchange for protons and the pump restores its resting state. ADP, adenosine diphosphate; ATP, adenosine triphosphate; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase.