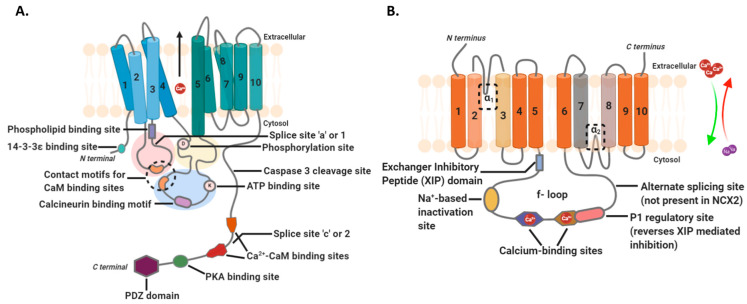
Figure 4.
Structures of intracellular Ca2+ efflux machinery. (A) Plasma membrane Ca2+-ATPase (PMCA) is comprised of cytosolic N- and C-termini, two Ca2+-binding sites (akin to SERCA; not shown here), and 10 transmembrane (TM) segments. Between the N- and C-tails lie three loop structures named like the domains in SERCA—A domain (red circle between TM 2–3), P domain (blue circle between TM 4–5), and N domain (yellow circle between TM 4–5) [34,36,37,38,39,40]. Various binding motifs and regulatory or interaction sites exist on each terminus and cytosolic domain with variations observed in their presence between PMCA isoforms and splice variants. Ca2+-bound calmodulin or Ca2+-CaM is a major regulator for all PMCA family members. The proximity of Ca2+-CaM binding sites on the C-tail to their corresponding motifs in A- and N-domains determine the open or closed states of the pump; (B) Sodium–Ca2+ exchanger (NCX) also has a ten transmembrane topology with N- and C-tails facing the extracellular space [42,43,44,45,49]. The cytosolic side consists of an f-loop (between TM 5–6) that has two Ca2+ ion binding sites, an alternate splicing site, a regulatory site, a sodium ion-dependent inactivation site, and an inhibitory domain. The structure also has two conserved alpha-helical repeats (α1 and α2) that influence ion binding and transport. ATP, adenosine triphosphate; CaM, calmodulin; NCX2, sodium-Ca2+ exchanger 2; PKA, protein kinase A; PDZ, a structural domain; XIP, exchanger inhibitory peptide.