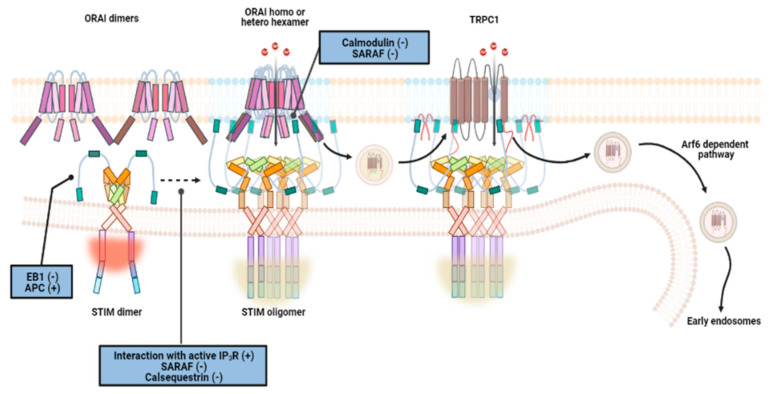
Figure 8.
Store-operated Ca2+ entry with TRPC1. Per the store-operated Ca2+ influx complex (SOCIC) model, Ca2+ influx mediated via STIM-ORAI complex has oscillatory patterns whereas bulk cellular entry of Ca2+ ions is mediated via TRPC1-STIM1 complex [122,124,146]. As described in the model, depletion of Ca2+ ions from the luminal space of the endoplasmic reticulum promotes oligomerization of STIM dimers on ER membrane [105,142,147,148]. Various STIM interacting proteins regulate this process including SARAF, calsequestrin, and activated IP3R. The symbol opposite each of these proteins in the figure depicts the outcome of their interaction with STIM on its oligomerization. As regulators of STIM insertion in the ER membrane, EB1 and APC indirectly influence STIM-ORAI puncta formation as well [136]. ORAI subunits also translocate from low lipid regions (yellow) on the plasma membrane to cluster into hexamers within cholesterol-rich lipid rafts (green). The ORAI-STIM puncta formation allows Ca2+ entry while simultaneously triggering detachment of TRPC1 from caveolae in vesicles and insertion into lipid-rich rafts on the plasma membrane [139,141]. From thereon, functional coupling between TRPC1 and STIM brings a large intracellular flow of Ca2+ ions. Activated ORAI hexamer also is purported to promote TRPC1 insertion into lipid rafts. After SOCIC-mediated Ca2+ ion influx, Rab4 coated vesicles transport TRPC1 via the Arf6 pathway to early endosomes for recycling [140,141]. APC, adenomatous polyposis coli; Arf6, ADP ribosylation factor 6; EB1, microtubule plus end-binding protein; SARAF, SOCE-associated regulatory factor.