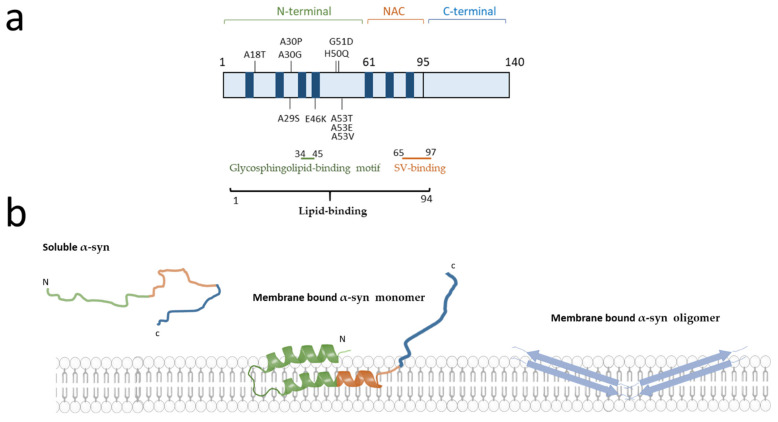
Figure 1.
Schematic representation of α-synuclein (α-syn) mutations and lipid binding regions. (a) Schematic representation of the domain structure of α-syn. The α-syn is composed of three domains: the N-terminal domain (green), the NAC domain (orange) and the C-terminal domain (blue). Four confirmed pathogenic autosomal dominant missense mutations (A30P, G51D, A53E, A53T) as well as six putatively pathogenic mutations (A18T, A29S, A30G, E46K, H50Q, A53V) are depicted [11]. In blue are represented the seven KTKEGV hexameric repeats spanning from the N-terminus to the non-amyloid β-component (NAC) domain. The lipid binding regions are represented by lines of different colours (black = lipid binding, green = glycosphingolipid-binding motif and red = synaptic vesicles (SV)). (b) Schematic representation of the different conformations of the α-syn. α-syn is present in the cytosol as unfolded monomer. Binding of α-syn to lipids induces a conformational change of α-syn N-terminal region, which acquires an α-helix secondary structure. The oligomers penetrate into the lipid bilayer with a β-sheet structure. The membrane image is adapted from Servier Medical Art (smart.servier.com, accessed on 19 July 2020) licensed under a Creative Commons Attribution 3.0 Unported License.