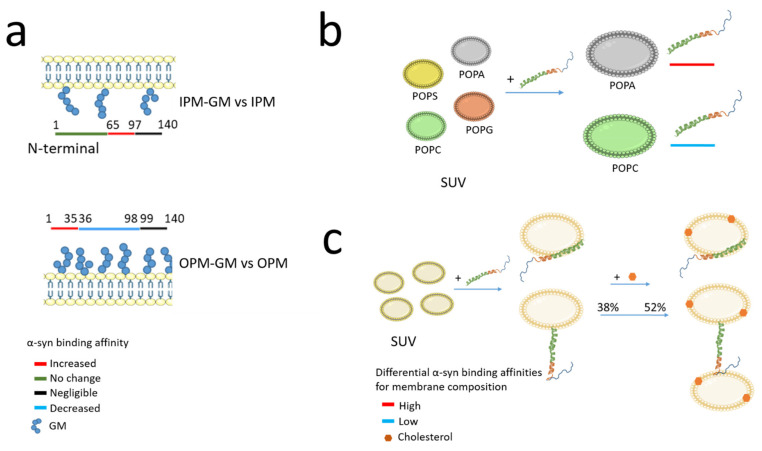
Figure 2.
Schematic representation of differential affinity of α-synuclein (α-syn) for the inner or outer plasma membrane (IPM or OPM) as well as for vesicles according to their lipid compositions. (a) Differential affinity of α-syn for IPM and OPM according to differences in the amount of gangliosides (GM): IPM-GM versus (vs) IPM or OPM-GM versus OPM as described by Man et al. (2021) [38]. (b) Differential affinity of α-syn for artificial vesicles based on their membrane composition. α-syn has a 60 times higher affinity for 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (POPA) than 1-palmitoyl-2-oleoyl-phosphatidyl-l-serine (POPS) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) and very low affinity for 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). (c) Effect of cholesterol on the conformation of α-syn. α-syn interacts with vesicles to promote fusion between 2 vesicles as described by Fusco et al. (2016) [61] and Man et al. (2020) [62]. Upon interaction with small unilamellar vesicles (SUV) composed of 1,2-dioleoyl-sn-glycero-3-phospho-ethanolamine (DOPE), 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), α-syn exists in multiple different conformational states. These include the α-helical state covering the 1–97 region (top) and a conformational state interacting with the membrane through N-terminal residues 1–25. It has been proposed by Man et al. (2020) that the presence of cholesterol in the SUV composition induces an increase in the proportion of α-syn with the conformational state described at the bottom from 38% to 52%, leading to the 65–97 region being available to interact with a second SUV [62]. This suggests that cholesterol promotes the docking of the vesicles-mediated by α-syn.