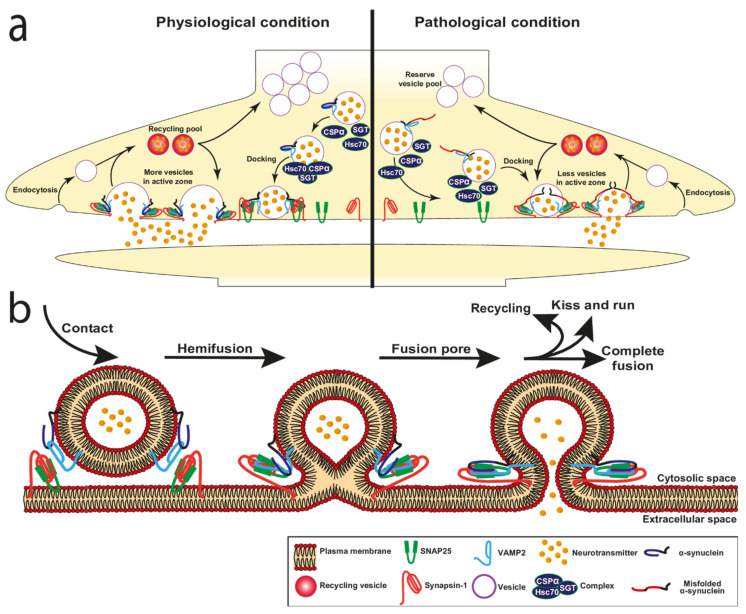
Figure 3.
Schematic hypothesis of the role of α-synuclein (α-syn) in exocytosis. (a) α-syn, under physiological condition (left panel), interacts with the soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) vesicle-associated membrane protein 2 (VAMP2) on the synaptic vesicles (SV) surface, drives the docking of the SV to the active zone and regulates the formation of the tripartite SNARE-complex. Others synaptic partners including synapsin-1 and complexin act in the complex stabilisation. The SNARE-complex regulates the fusion of the SV with the synaptic membrane. After cargo release, the vesicles are recycled. Under pathological condition (right panel) aberrant forms of α-syn have a stronger binding affinity for VAMP2. The reduced availability of unbound VAMP2 molecules inhibits the SNARE complex formation and reduces the number of vesicles in the active zone. (b) α-syn actively participates in exocytosis by regulating SNARE complex formation and vesicle fusion events. Indeed, α-syn favours dilatation/closure of the fusion pore as well as regulates the kiss and run exocytosis. SNAP25 = synaptosome associated protein 25, CSPα = cysteine-string protein-α, Hsc70 = heat shock cognate 70, SGT = small glutamine-rich tetratricopeptide repeat-containing protein α.