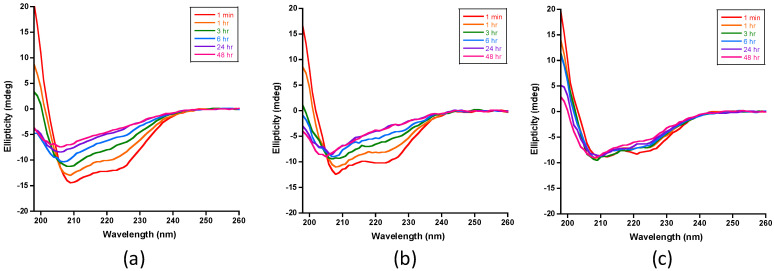
Figure 4.
CD spectra of IDE-dependent degradation of insulin in the absence and presence of polyphenols at 37 °C. (a) Spectra recorded for insulin digests that do not contain polyphenols (control samples) show the progressive loss on insulin’s helical CD signals at 222, 208, and 198 nm, consistent with the loss of α-helical structure. (b) Spectra recorded for insulin digests in the presence of resveratrol were similar to the spectra recorded for the control samples, indicating that the polyphenol had no effect on IDE’s activity toward insulin. (c) Spectra recorded for insulin digests in the presence of EGCG showed that the helical signals of insulin persist, indicating that EGCG inhibits insulin proteolysis by IDE. The insulin concentration in all samples was set at 20 μM in 50 mM Tris buffer (pH 7.4). The substrate-to-enzyme molar ratio and the polyphenol-to-insulin molar ratio were set to 100:1 and 2:1, respectively. All samples were kept at 37 °C in between spectral acquisition.