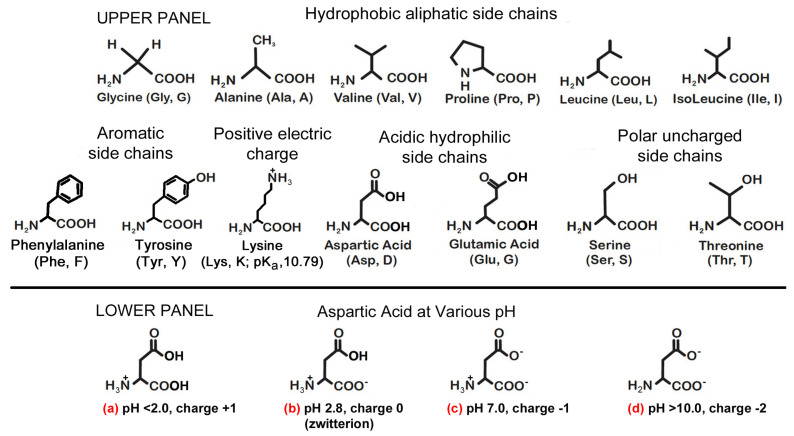
Figure 1.
Upper panel: shows thirteen amino acids that were thought to be present during the chemical evolution of life as reported in Table 1 column (g) [14]. Lower panel: shows aspartic acid has net protonated amide () side chains in (a–c) at various pH including a zwitterion (b) and a zero charge at pH 2.8. It has been reported that both aspartic acid and glutamic acid are particularly relevant in 65% of catalyst residues because of their ionic charges, remembering that it is the interplay of electrons which brings about reactions—cf the three hydrophobic amino acids used in Ikehara’s experiment Gly > Ala > Val. Likewise, serine and threonine (with their polar, uncharged, side chains), and tyrosine are also important in 27% of catalyst residues. To complete the comparison, hydrophobic amino acids (Gly, Ala, Val, Pro, Leu, and Ile) feature only 8% of the time at the active sites of enzymes [16,18]. In (d) aspartic acid represents as having overall charge of −2 when the pH exceeds 10.0.