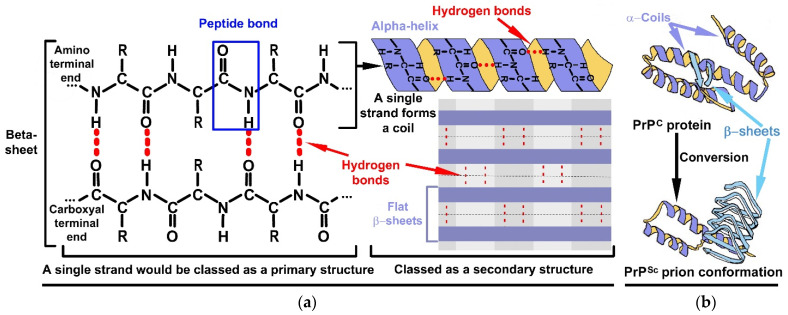
Figure 2.
(a) Formation of secondary level structures in the form of α-helices and β-sheets. The red dotted lines are an indication of the presence of hydrogen bonds between two peptide strands. The amino acids: glycine, alanine, aspartate and proline are widely distributed in α-helices; valine, isoleucine and tryptophan are commonly found in β-sheets [3]. Noting that tryptophan is not included in the top thirteen prebiotic amino acids indicated in Table 1. This raises two possibilities. Firstly, that initial proteins were much “simpler”, being made from only the thirteen amino acids. Secondly, that tryptophan is absent in the chemical inventory of comets [14] and was, thus, acquired later during the chemical evolution, meaning that they are probably biogenic in nature [18]. (b) within the context of PrPs, the PrPScs precipitate out into tightly packed tertiary structures which means they are able to withstand harsh environmental conditions when compared to RNA shapes. This figure also depicts that PrPC is more α-helices orientated than β-sheets. This situation is reversed in PrPSc.