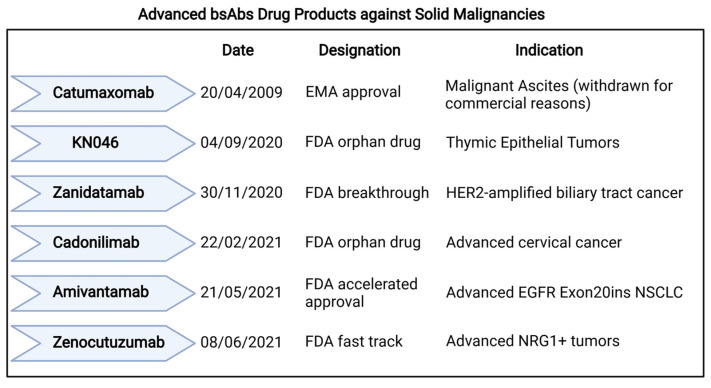
Figure 3.
Advanced bsAbs Drug Products against Solid Malignancies. The figure depicts bsAbs receiving regulatory designations for their use in solid malignancies. Designations included, approval, accelerated approval, fast track, breakthrough, orphan drug. EMA, European Medical Agency; FDA, Food and Drug Administration; HER2, human epidermal growth factor; EGFR, Epidermal Growth Factor Receptor; NSCLC, Non-Small Cell Lung Cancer; NRG1, Neuregulin 1. Created with BioRender.com.