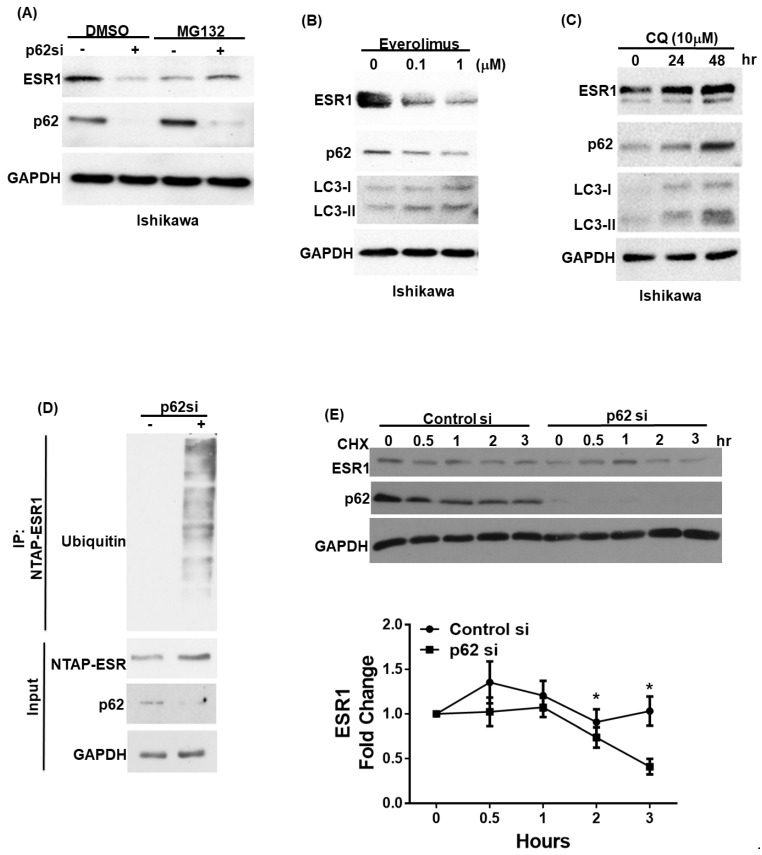
Figure 2.
p62 and estrogen receptor α(ESR1) expression levels are reciprocally interrelated. (A) Ishikawa cells were transfected with a control vector or p62 siRNA for 72 h. Subsequently, they were harvested after exposure to either DMSO or a proteasome inhibitor (MG132; concentration: 10 μM) for 5 h. Detection of endogenous ESR1, p62, and GAPDH was performed using specific antibodies. Ishikawa cells were treated with either (B) the mTOR inhibitor everolimus to promote p62 degradation or (C) the autophagy inhibitor chloroquine (CQ) to induce p62 accumulation. ESR1, p62, LC3 and GAPDH were detected by Western blot. (D) NTPA-ESR1 (CBP tag) was transfected into 293 cells with and without silenced p62 siRNA expression and harvested after treatment with DMSO or MG132 (10 μM) for 5 h. NTAP-ESR1 was precipitated with streptavidin beads, whereas ubiquitin-labeled proteins were identified using an anti-ubiquitin antibody. Anti-CBP tag and GAPDH served as control inputs. An antibody raised against p62 was used to confirm the efficiency of p62 silencing. (E) Ishikawa cells with and without silenced p62 were harvested at the reported time points in presence of the protein synthesis inhibitor cycloheximide (CHX). Detection of endogenous ESR1 and p62 was performed using specific antibodies. GAPDH served as the loading control followed by normalization for ESR1 levels. The fold changes in ESR1 protein levels are reported in the lower panel. Results are expressed as means ± standard errors of the mean from three independent experiments; * p < 0.05.