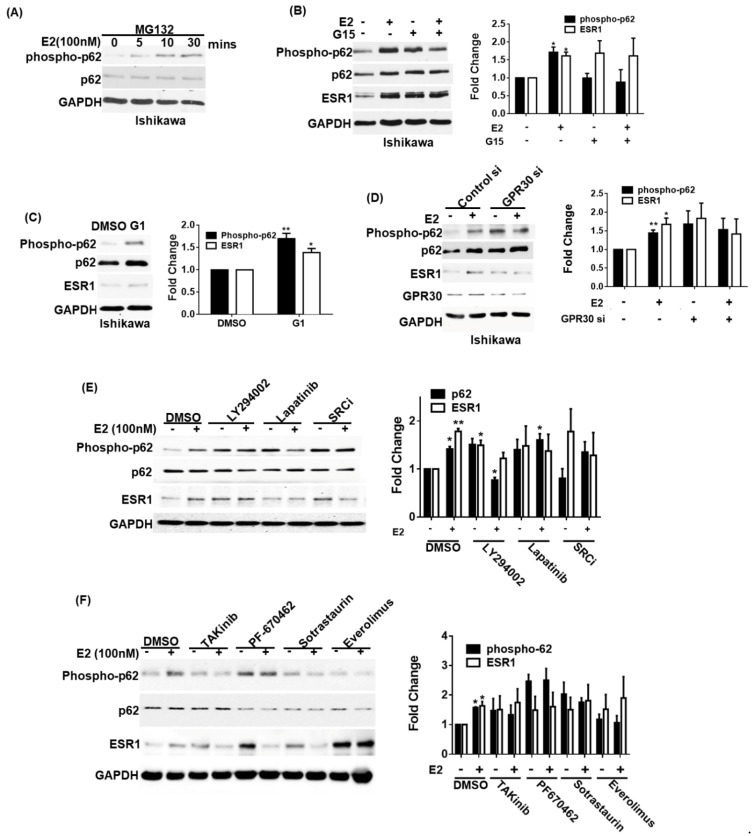
Figure 3.
Activation of GPR30 by 17β-estradiol promotes p62 phosphorylation and estrogen receptor α (ESR1 expression). (A) Ishikawa cells were pretreated overnight with the proteasome inhibitor MG132 (concentration: 5 μM) in phenol red-free OPTI-MEM medium. After harvesting, cells were exposed to 17β-estradiol (100 nM) at the reported time points. Phosphorylated and total p62 were detected using specific antibodies; endogenous GAPDH served as a loading control. (B) A pharmacological GPR30 antagonist (G15) inhibited 17β-estradiol-induced p62 phosphorylation and ESR1 expression. Ishikawa cells were starved overnight in presence of the proteasome inhibitor MG132 (concentration: 5 μM) in phenol red-free OPTI-MEM medium. On the next day, cells were pretreated with either G15 (100 nM) or a vehicle for 30 min and subsequently exposed to 17β-estradiol (100 nM) or a vehicle for 30 min. Protein levels of phosphorylated and total p62, ESR1, and GAPDH were examined with Western blot. Results for phosphorylated p62 and ESR1 were normalized for endogenous p62 and GAPDH expression levels. The fold changes for phosphorylated p62 and ESR1 protein levels are reported in the right panel. (C) A pharmacological GPR30 agonist (G1) enhanced p62 phosphorylation and ESR1 expression. Ishikawa cells were starved overnight in presence of the proteasome inhibitor MG132 (concentration: 5 μM) in phenol red-free OPTI-MEM medium and subsequently treated with G1 (10 nM). Protein levels of phosphorylated and total p62, ESR1, and GAPDH were examined with Western blot. Results for phosphorylated p62 and ESR1 were normalized for endogenous p62 and GAPDH expression levels. The fold changes for phosphorylated p62 and ESR1 protein levels are reported in the right panel. (D) Ishikawa cells were transfected with control or GPR30 siRNA for 72 h and subsequently exposed to 17β-estradiol (100 nM) or a vehicle for 30 min. Protein levels of phosphorylated and total p62, ESR1, and GAPDH were examined with Western blot. Results for phosphorylated p62 and ESR1 were normalized for endogenous p62 and GAPDH expression levels. The fold changes for phosphorylated p62 and ESR1 protein levels are reported in the left panel. (E,F) GPR30 downstream signaling pathways involved in p62 phosphorylation and ESR1 expression. Ishikawa cells were starved overnight in presence of the proteasome inhibitor MG132 (concentration: 5 μM) in phenol red-free OPTI-MEM medium and subsequently treated for 30 min with the following compounds: a PI3K inhibitor (LY294002), an EGF receptor inhibitor (lapatinib), a SRC inhibitor (SRCi), a TAK inhibitor (takinib), a casein kinase inhibitor (PF-670462), a PKC inhibitor (sotrastaurin), and an mTOR inhibitor (everolimus). Protein levels of phosphorylated and total p62, ESR1, and GAPDH were examined with Western blot. Results for phosphorylated p62 and ESR1 were normalized for endogenous p62 and GAPDH expression levels. The protein levels in vehicle control absence of E2 treatment as one. Results are expressed as mean ± standard errors from three independent experiments. Statistical significance was calculated with Student’s t-test * p < 0.05. ** p < 0.01.