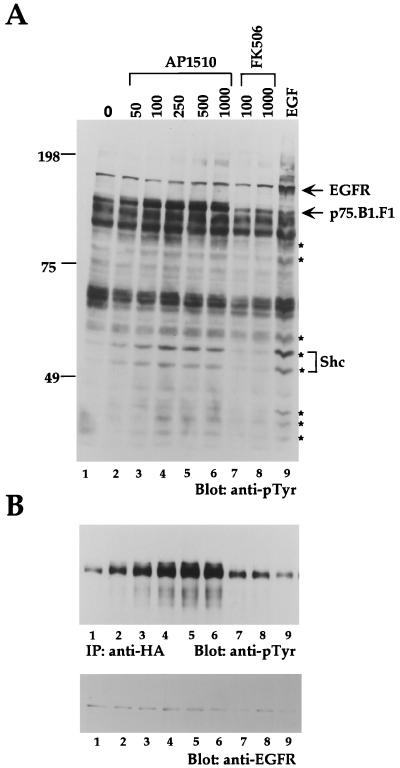
FIG. 2.
Dimerization of ErbB1 cytoplasmic domain with synthetic ligands results in a dose-dependent stimulation of receptor and substrate phosphorylation. (A) Rat1 fibroblasts expressing ErbB1 fused to one copy of FKBP (p75.B1.F1.HA) were stimulated with increasing amounts of AP1510 (nanomolar) (lanes 2 to 6) or FK506 (lanes 7 and 8) for 15 min or stimulated with 50 ng of EGF per ml for 5 min. Cell lysates were collected, and 45 μg of protein was resolved and immunoblotted with anti-phosphotyrosine (anti-pTyr) antibodies. The blot was stripped and reprobed with anti-Shc antibodies, and the p46 and p52 isoforms of Shc and other cellular proteins that were tyrosine phosphorylated by ligand stimulation are indicated by asterisks. (B) Seven hundred micrograms of lysate was used for immunoprecipitation with anti-HA antibodies and immunoblotted with anti-pTyr (upper panel). The anti-pTyr blot was subsequently stripped and reprobed with anti-EGFR antibodies (lower panel).