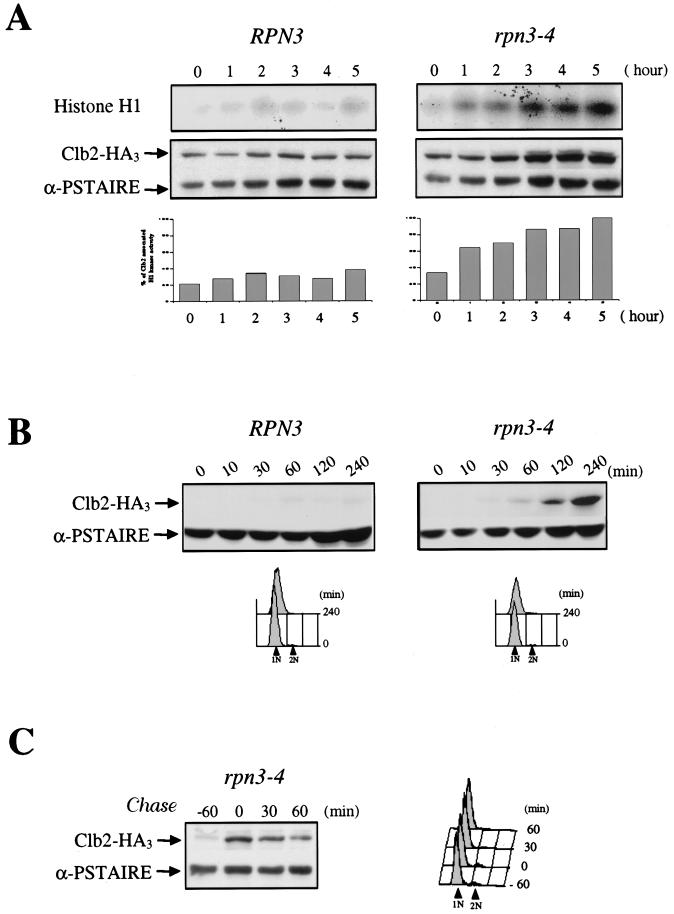
FIG. 4.
rpn3-4 mutants arrest with high levels of Clb2 and Clb2-associated histone H1 kinase activity and are defective in APC-dependent degradation of Clb2. (A) Wild-type YE44 (RPN3) cells and YE102 mutant (rpn3-4) cells carrying an HA-tagged CLB2 gene were grown and arrested as described in the legend to Fig. 3A. At hourly intervals, cell samples were recovered and Clb2-associated histone H1 kinase activity was determined by immunoprecipitation with an anti-HA antibody (upper panel; Histone H1). The same extracts were also analyzed by Western blotting with an anti-HA antibody to determine their Clb2 levels (lower panel; Clb2-HA3). Cdc28 immunodetected with an anti-PSTAIRE monoclonal antibody was used as a loading control (lower panel; α-PSTAIRE). Histograms represents quantification of the histone H1 kinase activity (percentage of Clb2-associated H1 kinase activity). (B) Accumulation of Clb2 in G1-arrested rpn3 mutant cells. YE106 (RPN3) cells and YE107 mutant (rpn3-4) cells harboring an HA-tagged CLB2 gene under the control of the inducible GAL1 promoter were grown to the early log phase in YEPR medium, arrested in G1 with α-factor for 2.5 h, and shifted to 37°C for an additional hour to inactivate the rpn3-4 gene product. At time zero, HA-tagged Clb2 expression was induced by adding galactose, and cell samples were collected at the indicated times for immunoblot analysis with an anti-HA antibody (Clb2-HA3). As in panel A, an immunoblot with an anti-PSTAIRE monoclonal antibody was used as a loading control (α-PSTAIRE). The effectiveness of the α-factor-induced G1 arrest in both experiments was assessed by FACScan analysis. 1N and 2N indicate cells with unreplicated and fully replicated nuclear DNA, respectively. (C) Clb2 is strongly stabilized in the rpn3 mutant. In an experiment similar to that shown in panel B, Clb2 expression was transiently induced with galactose for 60 min in G1-arrested YE107 (rpn3-4) cells and then repressed by transfer to prewarmed glucose-containing medium still in the presence of the pheromone. Cdc28 (α-PSTAIRE) is shown as a loading control.