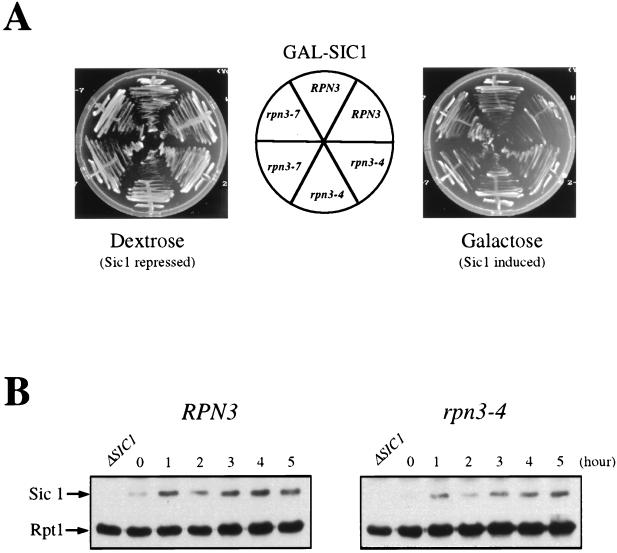
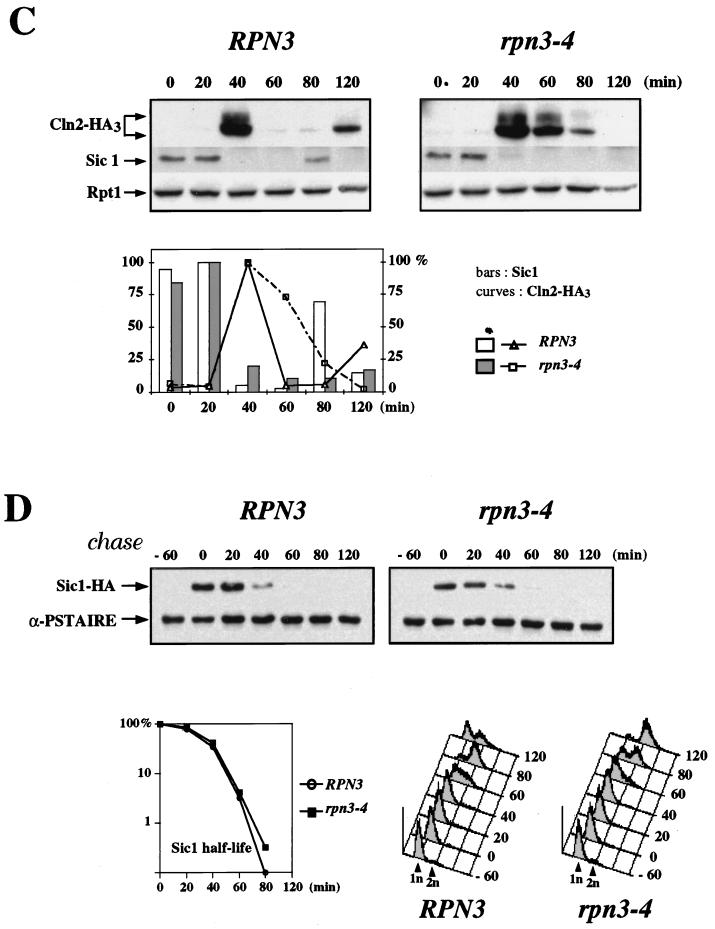
FIG. 8.
Sic1 degradation in an rpn3 mutant. (A) rpn3 mutants tolerate high levels of Sic1. Wild-type YE46 (RPN3) cells and rpn3 mutant YE100 and YE101 (rpn3-4 and rpn3-7) cells were transformed with a centromeric plasmid carrying an HA-tagged SIC1 gene under the control of the GAL1 promoter (YCpG-SIC1). Two independent transformants of each strain were streaked on selective medium containing either dextrose or galactose as a carbon source. Plates were photographed after 3 days of incubation at 30°C. (B) Sic1 levels in an arrested rpn3 mutant. Wild-type YE46 (RPN3) cells and rpn3 mutant YE100 (rpn3-4) cells were grown to the early log phase at 25°C and shifted to 37°C. Cells taken before (lane 0) and at hourly intervals after the shift were subjected to immunoblotting analysis with anti-Sic1 and anti-Rpt1 antisera. An extract from a SIC1-disrupted strain (ΔSIC1) was run in parallel as a control for the specificity of the anti-Sic1 antiserum. (C) Sic1 protein levels in synchronized rpn3 mutant cells. Control YE112 (RPN3) cells and rpn3 mutant YE113 (rpn3-4) cells, both carrying an HA-tagged CLN2 allele, were grown at 25°C to the early log phase, synchronized in G1 with α-factor, and shifted to 37°C. After release from the G1 arrest at the restrictive temperature, samples were withdrawn at the indicated times and probed by immunoblotting for HA-tagged Cln2, Sic1, and Rpt1 (as a loading control) with an anti-HA antibody (Cln2-HA3), Sic1-specific antiserum, and Rpt1/Cim5-specific antiserum, respectively. A graphic representation of the Sic1 and HA-tagged Cln2 (Cln2-HA3) immunoreactivities obtained by densitometry is also presented. (D) Sic1 turnover in an rpn3 mutant. Control YE114 (RPN3) cells and rpn3 mutant YE115 (rpn3-4) cells, both with an integrated GAL1:SIC1(HA)1 construct, were grown at 25°C in YEPR medium to the early log phase, arrested in G1 with α-factor, and shifted to 37°C. Galactose was added for 60 min to induce Sic1 expression, and the cells were returned to prewarmed glucose-containing medium to shut off the GAL1 promoter. Samples withdrawn before (lane −60) and after (lane 0) Sic1 induction and at the indicated times following glucose repression were subjected to Western blot analysis with anti-HA (Sic1-HA) and anti-PSTAIRE (α-PSTAIRE) antibodies to monitor HA-tagged Sic1 and Cdc28 (as a loading control), respectively. Flow cytometric analysis of the corresponding samples is also presented for each strain. A graphic representation of the Sic1 half-life, as estimated by immunoblotting, is also presented.