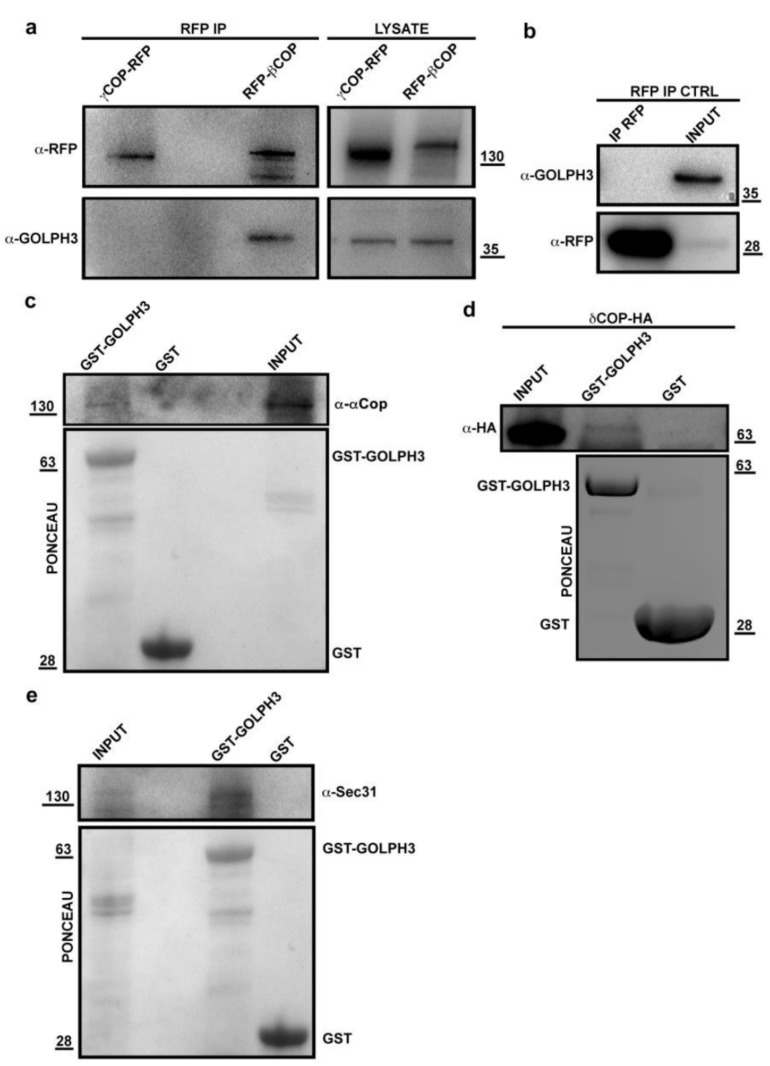
Figure 3.
dGOLPH3 interacts with COPI subunits and Sec31 protein. (a,b) dGOLPH3 protein coprecipitated with RFP–βCOP but not with γCOP-RFP and RFP. Protein extracts from testes expressing RFP–βCOP, γCOP-RFP (a) and RFP (RFP IP CTRL) (b), were immunoprecipitated with RFP-trap beads (α-RFP) and blotted for either RFP or dGOLPH3. 4% of the total lysates and one third of the IP were loaded and probed with the indicated antibodies. (c–e) GST pull-down to test dGOLPH3 interaction with αCOP (c), δCOP (d) and Sec31 (e) proteins. (c) Bacterially expressed GST and GST-dGOLPH3 were purified by Gluthatione-Sepharose beads, incubated with testis protein extracts from Oregon-R males and blotted for αCOP protein. (d) Bacterially expressed GST and GST-GOLPH3 were purified by Gluthatione-Sepharose beads, incubated with testis protein extracts from males expressing δCOP-HA. (e) Bacterially expressed GST-dGOLPH3 and GST purified by Gluthatione-Sepharose beads were incubated with testis protein extracts from Oregon-R males and blotted for Sec31. Ponceau staining in (c–e) is shown as a loading control. 2% of the input and 25% of the pull-downs were loaded and probed with the indicated antibody. Molecular masses in (a–e), expressed in kilodaltons.