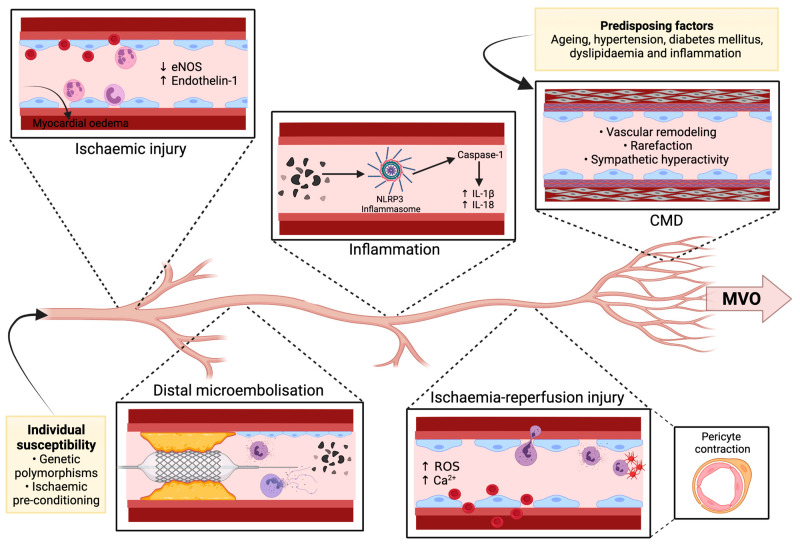
Figure 1.
Pathophysiology of MVO. Various genetic polymorphisms and the absence of ischaemic pre-conditioning predispose coronary microcirculation to injury. Plaque disruption, whether spontaneous or PCI-induced, results in distal microembolisation of cellular debris and augmentation of the inflammatory cascade, particularly neutrophil activation. Ischaemic injury to the microcirculation causes endothelial damage and extravasation of erythrocytes and inflammatory cells, resulting in myocardial oedema and luminal narrowing. Moreover, ischaemic injury promotes vasoconstriction by decreasing eNOS release and increasing endothelin-1 release, further limiting microvascular flow. Triggered by inflammatory debris, the NLRP3 inflammasome activates caspase-1 thereby mediating the cleavage of proIL-1b and proIL-18 into their active forms. Reperfusion after a prolonged ischaemic period further potentiates the inflammatory response by augmenting leukocyte recruitment, ROS generation, neutrophil-platelet aggregate formation, and calcium release. Ischaemia-reperfusion injury also induces pericyte contraction and intramyocardial haemorrhage which further limit microvascular flow. Increasing age, hypertension, diabetes mellitus, dyslipidaemia, and inflammation all predispose to CMD, which is characterised by perivascular fibrosis, smooth muscle cell and endothelial dysfunction, vascular remodelling, rarefaction, and sympathetic hyperactivity. These interdependent mechanisms form the underlying pathogenesis of MVO. Abbreviations: Ca2+, Calcium; CMD, Coronary microvascular dysfunction; eNOS, Endothelial nitric oxide synthase; MVO, Microvascular obstruction; ROS, Reactive oxygen species.