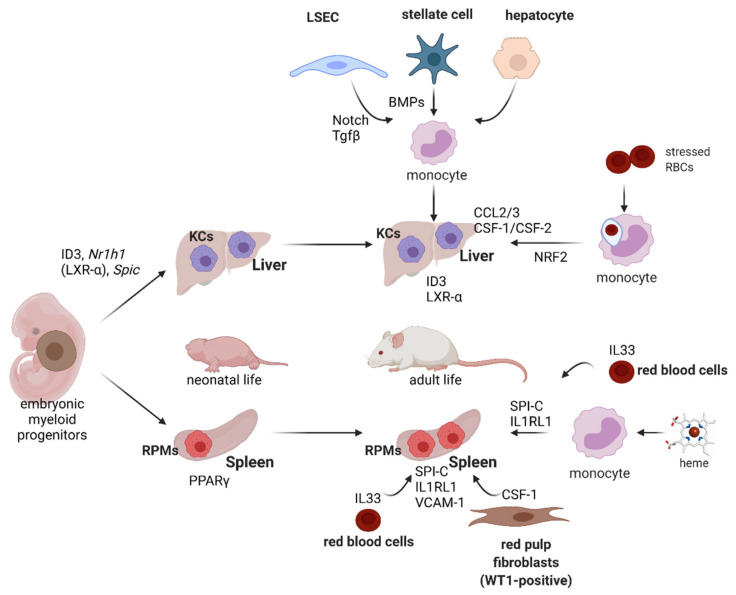
Figure 5.
Pathways that imprint KCs’ and RPMs’ identity and enable plasticity of iron-recycling cells. RPMs’ neonatal expansion depends on PPARγ, whereas in adult life, their identity and numbers are maintained by SPI-C, VCAM-1, the IL33–IL1RL1 axis and CSF1 released by red pulp fibroblasts. Upon hemolytic stress, differentiation of monocytes into RPM-like cells is mediated by Spi-c induction due to heme loading and IL33 signaling. KC development is controlled mainly by ID3 and LXR-α. Differentiation of monocytes into functional KCs in emptied KC niche requires interaction with liver sinusoidal endothelial cells (LSECs), hepatic stellate cells and hepatocytes and soluble factors that are secreted by these cells, including Notch ligands, TGF-β and BMPs. Under erythrolytic stress, blood monocytes were shown to engulf stressed RBCs and differentiate into KC-like cells, which is driven by the chemotactic factors present in the liver and intrinsic NRF2 signaling in monocytes.