Abstract
Objective:
To determine whether priming with 1 or 25Hz repetitive transcranial magnetic stimulation (rTMS) will enhance the benefits from treadmill training up to 3 months postintervention in people with Parkinson disease (PD), and to evaluate the underlying changes in cortical excitability.
Methods:
This randomized double-blind, placebo-controlled trial was conducted between October 2016 and December 2018. Fifty-one participants with PD were randomized to receive 12 sessions of rTMS (25Hz, 1Hz, or sham) followed by treadmill training. All participants were assessed at baseline and 1 day, 1 month, and 3 months postintervention. Primary outcome was fastest walking speed, and secondary outcomes were timed up-and-go test (TUG), dual-task TUG (DT-TUG), motor section of the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III), and electrophysiological evaluation of cortical excitability by TMS.
Results:
The 1 and 25Hz rTMS groups produced a greater improvement in fastest walking speed at 1 day and 3 months postintervention than the sham group. Only the 1 and 25Hz rTMS groups sustained the improvements in TUG, and had a significant improvement in DT-TUG and MDS-UPDRS-III for up to 3 months. Behavioral improvements correlated with increased cortical silent period and short-interval intracortical inhibition in both groups receiving real rTMS.
Interpretation:
Priming with 1 and 25Hz rTMS can augment the benefits of treadmill training and lead to long-term motor improvement up to 3 months postintervention. The motor improvement at follow-up was associated with a normalization of cortical excitability, which in turn suggests an alteration of the homeostatic plasticity range. Rebalancing cortical excitability by rTMS appears critical for plasticity induction.
Introduction
Gait disturbance is one of the most debilitating symptoms of Parkinson disease (PD), which is characterized by slow speed, short steps, and reduced dual-task walking ability.1 Although L-dopa therapy can provide effective symptomatic relief for PD initially, some gait difficulties persist and become more complex and refractory to pharmaceutical treatment as the disease progresses.2 Gait disorders can lead to functional decline, increased risk of falls, and early institutionalization in PD. There is a need to find interventions that maximize patients’ gait performance and functional independence. Gait training with treadmill has shown efficacy in improving gait performance in patients with PD.3 Another promising nonpharmaceutical treatment modality, repetitive transcranial magnetic stimulation (rTMS), was found to be effective in improving walking speed in PD.4 Abnormal cortical excitability and brain activity are believed to underlie motor disturbances in PD.5,6 Reversing these abnormalities by rTMS may promote symptom relief and enhance functional recovery. However, the effects of both treadmill training and rTMS have been modest and short-lived.3,4 The limited long-term efficacy of these treatment modalities may be due to impaired plasticity in the primary motor cortex (M1) in PD.7 Beyond the use of rTMS or treadmill training, combination of both modalities led to a greater improvement in walking speed in patients with PD compared to treadmill training alone.8 Although this preliminary finding appears robust, long-term effects and the mechanisms underlying the improvement are not understood.
rTMS could modulate cortical excitability; specifically, high-frequency rTMS (ie, ≥5Hz) enhances motor cortex excitability,9 whereas low-frequency rTMS (≤1Hz) downregulates cortical excitability.10 The approach of neuromodulation offers a promising rationale in potentiating the efficacy of motor training by priming it with rTMS for a more permanent neuroplastic change. In healthy individuals, motor learning can be enhanced if cortical excitability is transiently increased in a process known as “gating.”11 Motor learning can also be facilitated if neuronal activity is lowered in the motor cortex prior to training by taking advantage of homeostatic metaplasticity.12,13 Although the enhanced effect induced by rTMS in the aforementioned study in PD8 may be caused by the increase in cortical excitability with high-frequency rTMS, it is plausible that low-frequency rTMS can also improve the training effect as the threshold for increasing cortical excitability is lowered. However, the use of low-frequency rTMS to prime motor training in PD has not been examined before. Therefore, the priming effect of low-frequency rTMS is unknown and the important factors that govern the priming effect by rTMS on subsequent motor learning in PD remain to be elucidated. In this study, we examined whether 1Hz or 25Hz rTMS was more effective than sham rTMS in augmenting the benefits of treadmill training in patients with PD at 1 month and 3 months postintervention. We also determined whether any improvement in behavioral outcomes produced by active rTMS treatment was mediated by a change in corticomotor excitability. We further explored the relative efficacy between 1 and 25Hz rTMS in priming treadmill training.
Subjects and Methods
Study Design
This randomized double-blind, placebo-controlled trial was carried out at a neurophysiology laboratory at the Hong Kong Polytechnic University, Hong Kong. Fifty-one participants with mild to moderate PD (mean age = 62.3 years, standard deviation = 6.0; 24 female) were recruited from the movement disorder clinics of 3 local hospitals and through the Hong Kong Parkinson’s Disease Association, a patient self-help group. Individuals were eligible if they were diagnosed with idiopathic PD by a neurologist, aged between 40 and 70 years, had been stable on antiparkinsonian medication, and could walk a distance of 30m independently. The exclusion criteria were diagnosis of neurological diseases other than PD, severe comorbidities known to interfere with participation in the study, dementia (Mini-Mental State Examination < 24),14 a history of psychiatric disorders, no recordable motor evoked potentials (MEPs) with TMS, the presence of dyskinesia or tremors that would disturb stimulation, and contraindications for TMS.15 The study was approved by the university’s ethics committee. All study participants provided written informed consent to participate in the study in accordance with the Declaration of Helsinki. The trial was registered on ClinicalTrials.gov (NCT02701647).
Randomization and Blinding
Participants were randomly assigned using a web-based computer software research randomizer conducted by a research team member who was not the principal assessor, to 1 of the 3 groups (1:1:1) which received either 1Hz (1Hz-TT), 25Hz (25Hz-TT), or sham rTMS (sham-TT) followed by 30-minute treadmill training for 12 sessions over 3 weeks. Treatment allocation was concealed from the study investigator and assessor using opaque envelopes. Participants were blinded to rTMS conditions and all were naive to rTMS before the study. Participants were informed that the stimulation during rTMS was subthreshold for muscle contraction, and this intensity was much lower than that of single-pulse TMS used during electrophysiological evaluation. Hence, no peripheral sensation should be expected.
Procedures
Repetitive Transcranial Magnetic Stimulation.
Intervention was delivered by a physiotherapist who is well trained in TMS. The experimental groups (1Hz-TT and 25Hz-TT) received either 1 or 25Hz rTMS to the leg area of bilateral M1 while seated using a 90mm double-cone coil connected to a magnetic stimulator (Magstim Company, Whitland, UK). The coil was placed at optimal position over M1 for eliciting MEPs in the targeted tibialis anterior muscle. The M1 contralateral to the tibialis anterior muscle on the more-affected side was treated first, followed by that of the less-affected side. Each participant in the 1Hz-TT and 25Hz-TT groups received a total of 1,200 rTMS pulses at 80% of resting motor threshold (RMT). RMT is defined as the lowest intensity required to elicit MEPs of >50μV in at least 5 of 10 consecutive trials while the target muscle is relaxed.15 The RMT was determined to the nearest 1% of the maximum stimulator output.16 Participants in the 1Hz-TT group received 600 rTMS pulses in 10 minutes on each hemisphere. For the 25Hz-TT group, 600 rTMS pulses were delivered to each hemisphere in 4-second trains with an intertrain interval of 50 seconds. Sham rTMS was applied with a disconnected coil and another active coil behind the participant to mimic true stimulation sound effects without brain stimulation.
Treadmill Training.
Immediately after rTMS, participants proceeded with 30 minutes of treadmill training while wearing a safety harness to prevent falls. Treadmill speed was increased at increments of 0.2km/h every 5 minutes as tolerated. The maximum speed achieved was then maintained for the rest of the session or adjusted as needed. Verbal feedback was given to encourage participants to walk with an upright posture and large strides. Maximum walking speed, total walking distance, and self-perceived physical exertion measured by the Borg scale were recorded for each session to monitor exercise intensity.
Outcome Measures
Participants were assessed at the same time of day at baseline and 1 day (Post), 1 month (Post1m), and 3 months (Post3m) postintervention. All assessments were conducted with patients in the “on” medication state (ie, after taking their customary antiparkinsonian medication and having good therapeutic effect). Additionally, cortical excitability measures including the slope of recruitment (recruitment curve [RC]) and cortical silent period (CSP) were assessed immediately after rTMS on day 1 of the intervention session.
Behavioral Assessment
The primary behavioral outcome measure was the change in the fastest walking speed, which is a direct measure of the training effect of the treadmill training where participants were instructed to walk at their maximal tolerable speed. In addition, walking at the fastest speed poses a challenge to patients with PD, as bradykinesia is one of the cardinal symptoms and more balance control is required for fast walking. Participants were instructed to walk for 14m at their fastest walking speed, and the time taken to cover the middle 10m was recorded to calculate the fastest walking speed. The average speed over the 3 trials was used for analysis. Secondary outcome measures included the timed up-and-go test (TUG), dual-task TUG (DT-TUG), and the motor section of the Movement Disorders Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III). TUG and DT-TUG were performed by instructing participants to stand up from a chair, walk 7m, turn around, walk back to the chair, and sit down. For DT-TUG, participants were also required to perform a series of 3 subtraction tasks simultaneously. Completion time was captured using the Mobility Lab (APDM Wearable Technologies, Portland, OR).17 MDS-UPDRSIII was used to evaluate the severity of motor symptoms of PD.18 It comprises 27 items, including tremor, rigidity, bradykinesia, postural instability, and gait performance. Each item is scored from 0 to 4, with 0 indicating no disability and 4 indicating maximum disability, with total score ranging from 0 to 132. Freezing of gait was evaluated using item 3.11 in MDS-UPDRS-III, with 0 indicating no freezing, 1 and 2 indicating slight and mild freezing of gait, and 3 and 4 indicating moderate and severe freezing of gait.
Transcranial Magnetic Stimulation Techniques and Electromyographic Recordings
Electrophysiological measures including the linear slope of the RC,19 CSP, and short-interval intracortical inhibition (SICI) were recorded from electromyography (EMG) of the tibialis anterior muscle of the more-affected side using silver-silver chlo-ride surface EMG electrodes placed in a belly-tendon montage. EMG signals were amplified and filtered (bandpass = 2Hz and 10kHz). The signal was digitized (1401; Cambridge Electrical Design, Cambridge, UK) and exported into a computer for offline analysis.
The slope of the RC reflects the neurophysiological strength of corticospinal projections to the target muscle.15 Ten TMS stimuli were applied in 10% steps from 100 to 160% of each participant’s RMT. The cutoff intensity was set at 75% of maximum stimulator output due to the discomfort perceived by the majority of participants. The peak-to-peak amplitudes of 10 MEPs at each stimulus intensity were averaged offline and normalized with the maximal muscle action potential, which was determined by supramaximal electrical stimulation of the fibular nerve. The average normalized MEPs were plotted against stimulation intensity to obtain the linear slope of RC.
CSP is an interruption of EMG activity in a contracting muscle after a TMS trigger. It is largely due to an inhibitory mechanism originating from the motor cortex and is likely mediated by γ-aminobutyric acid type B (GABAB) receptors.15 To elicit CSP, stimulation intensities of 130 to 150% of RMT are most commonly used in the literature to ensure that the CSP durations reflect intracortical inhibition.20 The present study used 130% RMT, because stimulation intensity >130% RMT could lead to ceiling effects, which could preclude the ability to observe CSP changes after an intervention.21 Participants were instructed to perform a 20% isometric maximal contraction of tibialis anterior muscle while 10 suprathreshold TMS stimuli (ie, 130% RMT) were delivered. The duration of CSP was determined as the period between the onset of the MEP and the return to baseline EMG activity measured 100 milliseconds before TMS stimulus.22 Each CSP duration was determined, and the mean value of 10 CSPs was used for analysis. A longer CSP indicates greater intracortical inhibition.
SICI is another measure of the intracortical inhibition mediated by GABAA receptors.15 It is measured using a paired-pulse TMS paradigm according to Kujirai’s procedure.23 A test pulse was adjusted to produce MEPs of at least 0.5mV and was delivered after a conditioning pulse set at 80% RMT with an interstimulus interval of 2 milliseconds.15 A previous study indicated that the maximum suppression of the test response occurred with the conditioning pulse set at 80% RMT.23 Ten conditioned MEPs and 10 unconditioned MEPs were obtained in a random order and were averaged for each condition. SICI values are expressed as a percentage of the unconditioned test MEP amplitude. Values < 100% reflect inhibition, and a lower percentage for SICI denotes greater intracortical inhibition.
Statistical Analysis
All statistical analyses were performed using SPSS version 20.0 software (IBM, Armonk, NY). Our analysis included all participants who had at least one post-training assessment. If there were missing follow-up data, last observation carried forward was adopted. All outcome measures were separately analyzed with a 3 × 4 two-factor repeated-measures analysis of variance (ANOVA) with one within-subject factor, that is, assessment interval (baseline, Post, Post1m, Post3m), and 1 between-subject factor, that is, intervention group (1Hz-TT, 25Hz-TT, sham-TT). When there was an interaction effect between group and time, a 1-way repeated-measure ANOVA was followed by post hoc pairwise comparison with Bonferroni adjustment to achieve multiple comparisons of 3 time points for each treatment group. A between-group analysis of changes from the baseline was performed using a 1-way ANOVA, and Tukey adjustment was done for multiple comparisons of the change scores for the 3 intervention groups at each postintervention time point. Pearson correlation analysis was applied to establish the associations between the electrophysiological and behavioral measures. All statistical tests were 2-tailed with a 5% level of statistical significance. The sample size calculation is based on a previous study,8 considering 80% power and an effect size of 0.2 at a 5% level of significance; assuming 10% attrition, 17 subjects would be needed per group.
Results
Of 87 patients screened between October 19, 2016 and June 15, 2018, 51 participants were recruited and randomly assigned to 25Hz-TT, 1Hz-TT, or sham-TT, and 50 participants completed the study (Fig 1). Baseline demographic and clinical characteristics were similar among groups (Table 1). Five and 3 participants had slight and mild gait freezing, respectively, but none had moderate to severe freezing of gait, as assessed with item 3.11 of MDS-UPDRS-III. No participant had freezing of gait during the assessment sessions. Training dose and intensity received by each group were similar. The procedure was well tolerated by all participants, with no adverse effects reported. Changes in each behavioral and electrophysiological outcome across assessment intervals are presented in Table 2.
FIGURE 1:
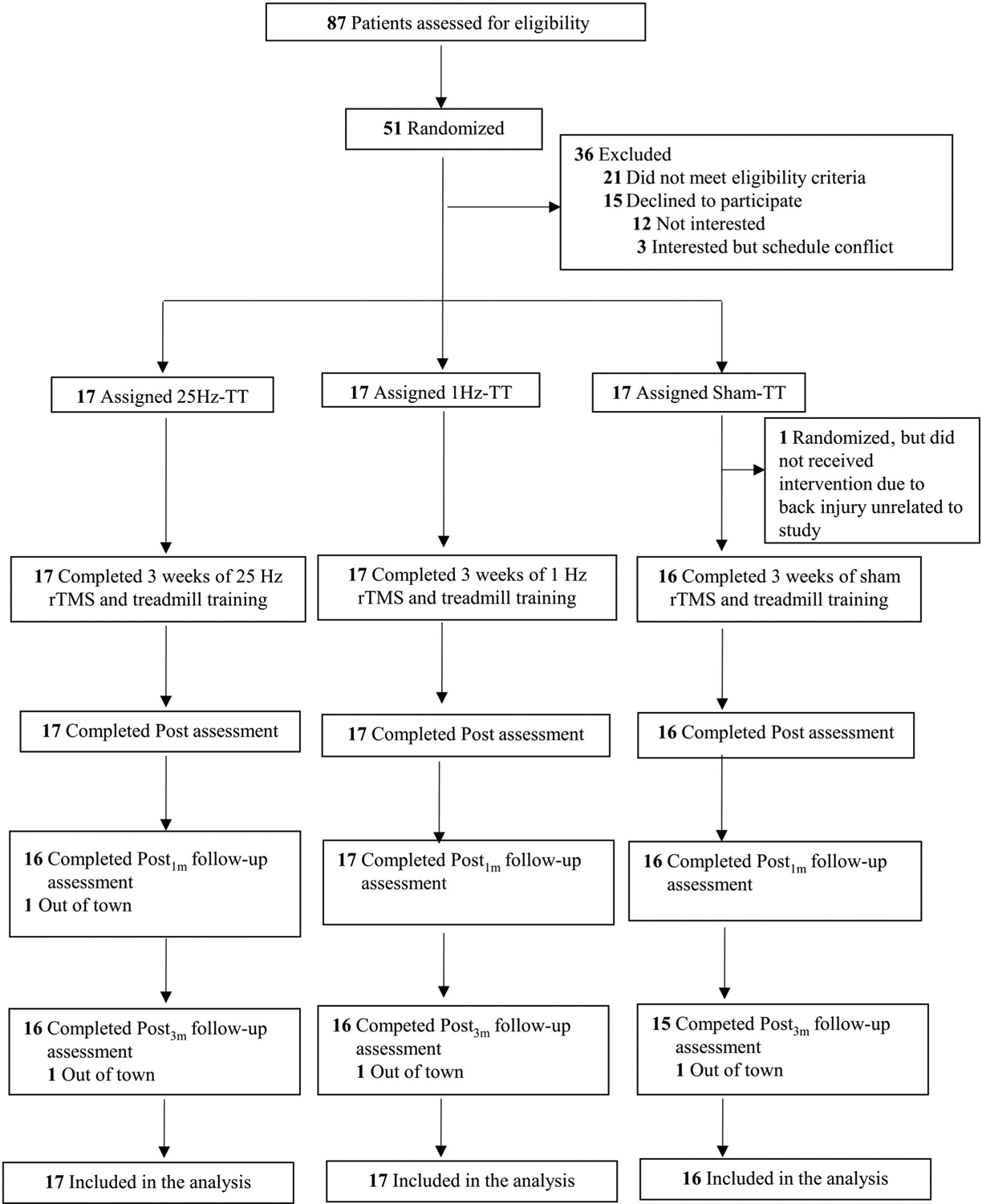
Trial flow chart. Post = 1 day postintervention; Post1m = 1 month postintervention; Post3m = 3 months postintervention; rTMS = repetitive transcranial magnetic stimulation; TT = treadmill training.
TABLE 1.
Baseline Clinical Findings and Cortical Excitability in the Participants Receiving Different Repetitive TMS Protocols
| Age, yr | 62.7 ± 6.8 | 62.1 ± 5.7 | 62.1 ± 5.7 | 0.959 |
| Men | 10 (59%) | 9 (53%) | 7 (44%) | 0.684 |
| Women | 7 (41%) | 8 (47%) | 9 (56%) | 0.684 |
| Disease measures | ||||
| LEDD, mg/day | 484.2 ± 336.4 | 512.5 ± 359.9 | 493.3 ± 523.9 | 0.979 |
| Disease duration, yr | 5.2 ± 3.4 | 7.5 ± 4.9 | 6.9 ± 3.3 | 0.220 |
| H&Y, range = 1–5 | 2.2 ± 0.3 | 2.2 ± 0.4 | 2.3 ± 0.3 | 0.670 |
| MDS-UPDRS-III, range = 0–132 | 27.9 ± 10.5 | 27.1 ± 9.6 | 29.7 ± 10.6 | 0.762 |
| Gait and mobility measures | ||||
| Fastest walking speed, cm·s−1 | 149.5 ± 24.7 | 153.1 ± 27.3 | 156.1 ± 30.2 | 0.788 |
| TUG, s | 20.8 ± 4.0 | 18.3 ± 2.5 | 19.3 ± 2.5 | 0.080 |
| DT-TUG, s | 27.9 ± 8.8 | 24.0 ± 7.0 | 25.5 ± 5.6 | 0.297 |
| Electrophysiological measures | ||||
| RMT, % TMS output intensity | 47.8 ± 8.5 | 47.5 ± 9.8 | 49.7 ± 7.9 | 0.746 |
| Slope of RC | 0.51 ± 0.30 | 0.53 ± 0.39 | 0.61 ± 0.32 | 0.659 |
| CSP, ms | 176.5 ± 50.0 | 164.6 ± 39.8 | 183.9 ± 38.9 | 0.451 |
| SICI, % | 56.4 ± 27.0 | 49.4 ± 24.1 | 62.2 ± 33.1 | 0.518 |
Values are mean ± standard deviation.
CSP = cortical silent period; DT = dual-task; H&Y = Hoehn & Yhar stage; LEDD = L-dopa equivalent daily dose; MDS-UPDRS-III = Part III (motor section) of Movement Disorders Society–Unified Parkinson’s Disease Rating Scale; RC = recruitment curve; RMT = resting motor threshold; SICI = short-interval intracortical inhibition; TMS = transcranial magnetic stimulation; TT = treadmill training (TT); TUG = timed up-and-go test.
TABLE 2.
Comparison of Behavioral and Electrophysiological Outcome Measures at Baseline, 1 Day, 1 Month, and 3 Months after Intervention Ended
| Behavioral | |||||||
| 25Hz | 149.5 ± 24.7 | 164.3 ± 26.3 | 167.8 ± 26.4 | 163.2 ± 31.0 | |||
| 1Hz | 153.1 ± 27.3 | 167.3 ± 34.0 | 170.6 ± 35.6 | 169.9 ± 38.7 | |||
| Sham | 156.1 ± 30.2 | 160.7 ± 33.3 | 164.6 ± 32.3 | 156.7 ± 28.0 | |||
| 25Hz | 20.8 ± 4.0 | 18.4 ± 2.8 | 17.7 ± 2.9 | 17.9 ± 2.8 | |||
| 1Hz | 18.3 ± 2.5 | 17.1 ± 2.7 | 17.1 ± 2.3 | 16.6 ± 2.3 | |||
| Sham | 19.3 ± 2.5 | 18.0 ± 2.1 | 17.9 ± 1.8 | 18.4 ± 2.1 | |||
| 25Hz | 27.9 ± 8.8 | 23.1 ± 5.6 | 21.8 ± 6.0 | 22.3 ± 6.0 | |||
| 1Hz | 24.0 ± 7.0 | 21.1 ± 5.2 | 20.6 ± 4.7 | 20.3 ± 4.6 | |||
| Sham | 25.5 ± 5.6 | 23.4 ± 4.0 | 23.9 ± 3.9 | 23.9 ± 3.9 | |||
| 25Hz | 27.9 ± 10.5 | 19.9 ± 8.5 | 20.8 ± 8.9 | 22.5 ± 10.1 | |||
| 1Hz | 27.1 ± 9.6 | 22.2 ± 7.5 | 22.5 ± 7.3 | 21.9 ± 7.8 | |||
| Sham | 29.7 ± 10.6 | 27.9 ± 9.0 | 27.9 ± 10.1 | 27.3 ± 8.0 | |||
| Electrophysiological | |||||||
| 25Hz | 176.5 ± 50.0 | 194.4 ± 53.2 | 190.1 ± 54.5 | 175.9 ± 43.2 | |||
| 1Hz | 164.6 ± 39.8 | 180.6 ± 39.1 | 160.1 ± 41.1 | 154.6 ± 46.0 | |||
| Sham | 183.9 ± 38.9 | 175.1 ± 46.6 | 189.2 ± 37.6 | 182.9 ± 35.0 | |||
| 25Hz | 56.4 ± 27.0 | 44.4 ± 23.0 | 42.0 ± 26.9 | 50.8 ± 22.8 | |||
| 1Hz | 49.4 ± 24.1 | 51.4 ± 28.2 | 62.6 ± 31.7 | 60.7 ± 39.2 | |||
| Sham | 62.2 ± 33.1 | 76.5 ± 49.8 | 89.8 ± 62.9 | 74.3 ± 30.2 | |||
| 25Hz | 0.51 ± 0.30 | 0.58 ± 0.36 | 0.70 ± 0.57 | 0.74 ± 0.47 | |||
| 1Hz | 0.53 ± 0.39 | 0.64 ± 0.37 | 0.63 ± 0.30 | 0.70 ± 0.41 | |||
| Sham | 0.61 ± 0.32 | 0.52 ± 0.40 | 0.59 ± 0.43 | 0.58 ± 0.45 |
Values are mean ± standard deviation.
CSP = cortical silent period; DT = dual-task; MDS-UPDRS-III = Part III (motor section) of Movement Disorders Society–Unified Parkinson’s Disease Rating Scale; pa = time effect; pb = group × time interaction, using 3 × 4 two-factor repeated-measures analysis of variance comparison; Post = 1 day postintervention; Post1m = 1 month postintervention; Post3m = 3 months postintervention; RC = recruitment curve; SICI = short-interval intracortical inhibition; TUG = timed up-and-go test.
Primary Outcome
After 3 weeks of intervention, all subject groups showed an improvement in fastest walking speed from Post to Post3m (time effect, F3,47 = 18.86, η2 = 0.29, p < 0.001; Table 2). The 25Hz-TT group increased fast walking speed by 14.8cm·s−1 at Post, 18.3cm·s−1 at Post1m, and 13.7cm·s−1 at Post3m. The 1Hz-TT group improved fast walking speed by 14.2cm·s−1 at Post, 17.6cm·s−1 at Post1m, and 16.8cm·s−1 at Post3m. The sham-TT improved fast walking speed by 4.6cm·s−1 at Post, 8.5cm·s−1 at Post1m, and 0.64cm·s−1 at Post3m. Between-group analysis for the changes from baselines revealed that 1Hz-TT had a trend of greater improvement than sham-TT at Post (by 9.6cm·s−1; 95% confidence interval [CI] = −0.6 to 19.9, p = 0.070), and the difference reached significance at Post3m (by 16.3cm·s−1; 95% CI = 3.1–29.5, p = 0.012; Fig 2A). 25Hz-TT had a marginally greater improvement than sham-TT at Post (by 10.2cm·s−1; 95% CI = −0.04 to 20.5, p = 0.051) and at Post3m (by 13.2cm·s−1; 95% CI = −0.03 to 26.4, p = 0.051). No difference was found when comparing 25Hz-TT with 1Hz-TT at any time point.
FIGURE 2:
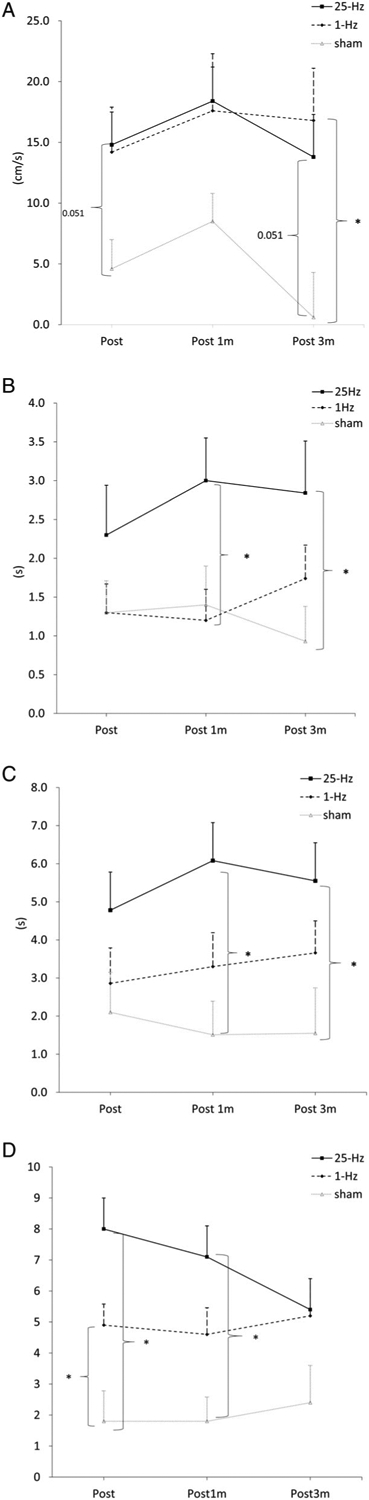
(A) Increase in fastest walking speed. (B) Reduction in timed up-and-go test (TUG) time. (C) Reduction in dual-task TUG time. (D) Reduction in motor section of the Movement Disorders Society–Unified Parkinson’s Disease Rating Scale scores. Post = 1 day postintervention; Post 1m = 1 month postintervention; Post 3m = 3 months postintervention. * denotes p < 0.05.
Secondary Outcomes
Significant interaction effects were found in TUG (time × group, F6,47 = 2.56, η2 = 0.10, p = 0.033), DT-TUG (time × group, F6,47 = 2.80, η2 = 0.11, p = 0.031), and MDS-UPDRS-III scores (time × group, F6,47 = 4.16, η2 = 0.15, p = 0.002; Table 2). For TUG time, all groups showed improvement at Post but only 1Hz-TT (p = 0.006; Table 3) and 25Hz-TT (p = 0.004; Table 3) sustained the improvement up to Post3m. Between-group comparison showed that 25Hz-TT had a greater improvement in TUG time than 1Hz-TT at Post1m (by −1.8 seconds; 95% CI = −3.4 to −0.2, p = 0.029) and sham-TT at Post3m (by −1.9 seconds; 95% CI = −3.7 to −0.1, p = 0.039; Fig 2B). Because the TUG test consists of a 7m walking component, exploratory analysis was performed to examine the time taken to complete the walking portion of TUG. Results showed that there was no group × time interaction effect and all subject groups had small although significant reductions in time spent on the walking component of TUG (time effect, F3,47 = 8.47, η2 = 0.15, p < 0.001; Tables S1 and S2, Supplementary Materials).
TABLE 3.
Mean Changes from Baseline and Within-Group Comparisons of Behavioral and Electrophysiological Outcome Measures between Baseline and 1 Day, 1 Month, and 3 Months after Intervention Ended
| 25Hz | 14.8 (6.7, 22.9) | 18.3 (9.9, 26.7) | 13.7 (3.3, 24.2) | ||||
| 1Hz | 14.2 (3.2, 25.2) | 17.6 (4.7, 30.4) | 16.8 (4.0, 29.6) | ||||
| Sham | 4.6 (−2.5, 11.8) | 8.5 (−5.9, 22.9) | 0.64 (−10.6, 11.9) | ||||
| 25Hz | −2.3 (−4.3, −0.4) | 0.015 | −3.0 (−4.7, −1.4) | <0.001 | −2.8 (−4.9, −0.8) | 0.004 | |
| 1Hz | −1.3 (−2.4, −0.2) | 0.020 | −1.2 (−2.4, 0.0) | 0.040 | −1.7 (−3.1, −0.4) | 0.006 | |
| Sham | −1.3 (−2.6, −0.1) | 0.038 | −1.4 (−2.9, 0.1) | 0.078 | −0.9 (−2.3, 0.4) | 0.344 | |
| 25Hz | −4.8 (−8.2, −1.4) | 0.004 | −6.1 (−9.5, −2.6) | <0.001 | −5.6 (−9.7, −1.4) | 0.006 | |
| 1Hz | −2.9 (−5.7, −0.1) | 0.043 | −3.3 (−6.0, −0.7) | 0.010 | −3.7 (−6.2, −1.1) | 0.003 | |
| Sham | −2.1 (−5.3, 1.1) | 0.373 | −1.5 (−4.2, 1.2) | 0.628 | −1.6 (−5.2, 2.1) | 1.000 | |
| 25Hz | −8.0 (−11.2, −4.8) | <0.001 | −7.1 (−9.9, −4.2) | <0.001 | −5.4 (−8.8, −1.9) | 0.002 | |
| 1Hz | −4.9 (−7.0, −2.9) | <0.001 | −4.6 (−7.2, −2.0) | <0.001 | −5.2 (−8.7, −1.6) | 0.003 | |
| Sham | −1.8 (−4.7, 1.2) | 0.568 | −1.8 (−4.2, 0.5) | 0.203 | −2.4 (−6.0, 1.1) | 0.335 | |
| 25Hz | 17.9 (−5.7, 41.5) | 0.260 | 13.6 (−12.0, 39.3) | 0.773 | −0.6 (−24.3, 23.1) | 1.000 | |
| 1Hz | 16.0 (−6.6, 38.5) | 0.295 | −4.5 (−23.5, 14.5) | 1.000 | −10.0 (−38.0, 18.1) | 1.000 | |
| Sham | −8.8 (−32.1, 14.6) | 1.000 | 5.3 (−11.8, 22.4) | 1.000 | −0.9 (−17.2, 15.4) | 1.000 | |
| 25Hz | −12.0 (−29.8, 5.7) | −14.4 (−35.9, 7.01) | −5.7 (−24.5, 13.2) | ||||
| 1Hz | −0.2 (−19.5, 19.1) | 13.3 (−8.7, 35.3) | 11.4 (−23.5, 46.3) | ||||
| Sham | 14.3 (−26.5, 55.1) | 27.5 (−22.7, 77.8) | 12.1 (−20.2, 44.5) | ||||
| 25Hz | 0.07 (−0.20, 0.34) | 0.19 (−0.25, 0.63) | 0.23 (−0.16, 0.62) | ||||
| 1Hz | 0.10 (−0.13, 0.34) | 0.10 (−0.13, 0.22) | 0.17 (−0.09, 0.42) | ||||
| Sham | −0.09 (−0.34, 0.16) | −0.03 (−0.23, 0.17) | −0.04 (−0.28, 0.20) |
Probability values are from post hoc analysis using one-way repeated-measures analysis of variance comparison with baseline, adjusted for multiple comparisons. Post hoc test was not performed for fastest walking speed, SICI, and slope of RC, because there was no significant group × time interaction. CI = confidence interval; CSP = cortical silent period; DT = dual-task; MDS-UPDRS-III = MDS-UPDRS-III = Part III (motor section) of Movement Disorders Society–Unified Parkinson Disease Rating Scale; NA = nonapplicable; Post = 1 day postintervention; Post1m = 1 month postintervention; Post3m = 3 months postintervention; RC = recruitment curve; SICI = short-interval intracortical inhibition; TUG = timed up-and-go test.
For DT-TUG time and MDS-UPDRS-III score, 1Hz-TT and 25Hz-TT but not sham-TT showed significant improvement at all time points (Table 3). For all groups, performance of cognitive tasks during DT-TUG did not decline and there were no differences among the groups. Between-group comparison showed that 25Hz-TT had a greater improvement in DT-TUG time than sham-TT at Post1m (by −4.6 seconds; 95% CI = −7.9 to −1.2, p = 0.006) and Post3m (by −4.0 seconds, 95% CI = −8.0 to −0.03, p = 0.048; Fig 2C). Both 25Hz-TT and 1Hz-TT outperformed sham-TT in reducing MDSUPDRS-III scores at Post (1Hz-TT, by −3.2; 95% CI = −6.4 to −0.02, p = 0.048; 25Hz-TT, by −6.3; 95% CI = −9.4. to −3.1, p < 0.001; Fig 2D); 25Hz-TT showed a trend of greater reduction in MDS-UPDRS-III scores than 1Hz-TT at Post (p = 0.056) and a significant greater reduction than sham-TT at Post1m (by −5.3; 95% CI = −8.2 to −2.3, p < 0.001; Fig 2D).
Electrophysiological Outcomes after a Single Session of rTMS on the First Day of Intervention
No significant interaction effect or time effect was found after a single session of rTMS on day 1 of the intervention for RC slope and CSP (Table S3, Supplementary Materials). A between-group comparison for the changes in the slope of RC and CSP from the baseline found no difference among the 3 intervention groups (Table S4, Supplementary Materials). No significant correlation was found between changes in CSP or RC slope after a single session of rTMS and the changes in any behavioral outcome measure for all subjects in both the short and long term (Table S5, Supplementary Materials).
Electrophysiological Outcomes after Completion of 3-Week Intervention
No intervention had a significant effect on the slope of RC at any assessment interval. For the CSP, a significant interaction effect (time × group, F6,46 = 2.61, η2 = 0.10, p = 0.020) was found; both 25Hz-TT and 1Hz-TT prolonged the CSP at Post, although the change did not reach statistical significance (Table 3). Between-group analysis revealed that 25Hz-TT had a greater prolongation of CSP than sham-TT at Post (by 28.1 milliseconds; 95% CI = 0.3–55.9, p = 0.047; Fig 3B). No significant change in SICI or interaction effect was evident; however, a trend of difference was found between 25Hz-TT and sham-TT at Post (by −26.4%; 95% CI = −54.5 to 1.6, p = 0.068), which reached significance at Post1m (by −42.0%; 95% CI = −75.9 to −8.1, p = 0.012; Fig 3C).
FIGURE 3:
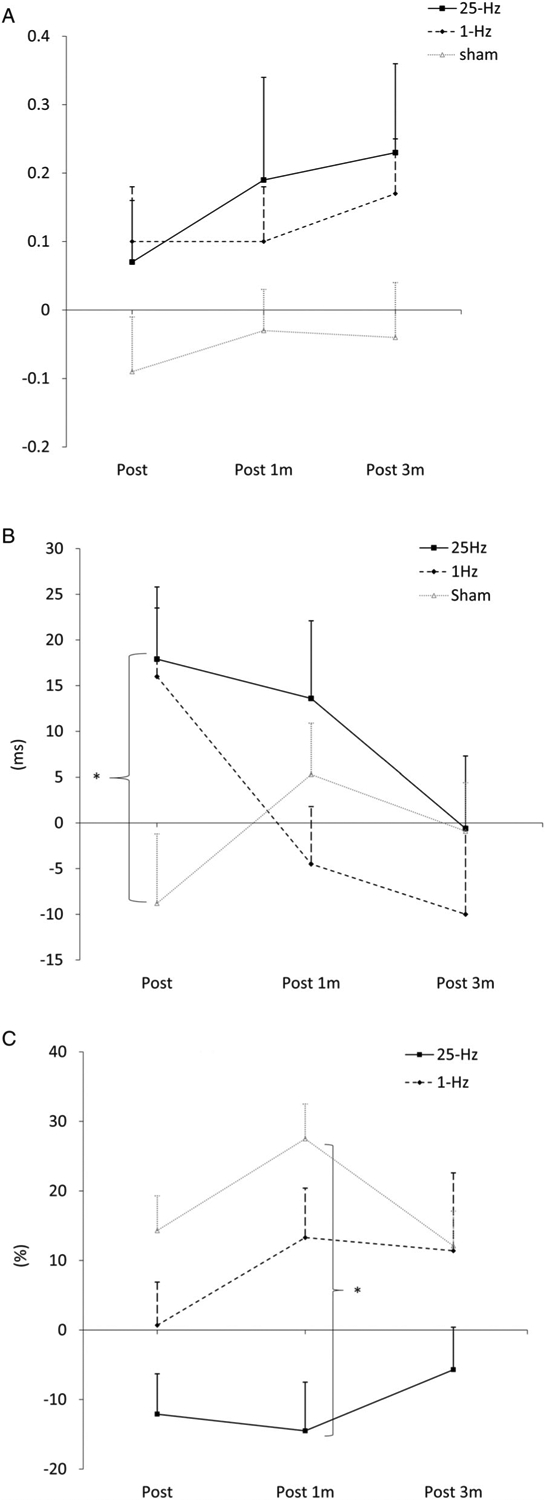
(A) Change in slope of recruitment curve. (B) Change in cortical silent period. (C) Change in short-interval intracortical inhibition. Post = 1 day postintervention; Post 1m = 1 month postintervention; Post 3m = 3 months postintervention. * denotes p < 0.05.
For 25Hz-TT, a greater inhibition in SICI at Post was associated with a greater reduction in MDS-UPDRSIII at Post1m (r = 0.524, p = 0.045), and a prolonged CSP duration was associated with a reduced TUG time at Post1m (r = −0.506, p = 0.038). For 1Hz-TT, a prolonged CSP was associated with a reduced MDS-UPDRS-III at Post1m (r = −0.560, p = 0.020) and Post3m (r = −0.491, p = 0.045) as well as reduced TUG time at Post3m (r = −0.551, p = 0.022). A prolonged CSP at Post1m was associated with reduced TUG time at Post3m (r = −0.504, p = 0.039). No significant correlation between behavioral and electrophysiological changes was found in sham-TT.
Discussion
Three main findings arose from our investigation. First, rTMS-primed treadmill training has greater long-term beneficial effects on walking performance, complex walking tasks, and motor symptoms in patients with PD. Second, our results showed that the improvement in behavioral measures correlated with the increase in intracortical inhibition in the 1Hz-TT and 25Hz-TT groups. Third, 1Hz and 25Hz rTMS had a similar beneficial effect in promoting gait training with a treadmill in patients with PD. The implication of these findings is 2-fold: (1) priming treadmill training with high- or low-frequency rTMS induced long-lasting therapeutic effects in patients with PD; and (2) long-lasting clinical benefits that were accompanied by a normalization of brain excitability could be due to metaplasticity, possibly by expansion of the homeostatic range.
Effect on Trained Motor Function
Motor learning in patients with PD has been found to be less efficient than in healthy individuals. Specifically, there is a deficiency in the consolidation and retention of new skills,24 as well as marked difficulty in shifting to the automatic stage of performing learned motor tasks.25 In the present study, treadmill training resulted in improvement in fastest walking speed in all participants. The increases from the baselines of the 25Hz-TT group at Post1m (ie, 18.3cm·s−1) and 1Hz-TT group at Post1m and at Post3m (ie, 17.6cm·s−1 and 16.8cm·s−1) reached the minimal detectable change of 15.7cm·s−1 in community-dwelling older adults with PD.26 In addition, these observed changes exceeded 10cm·s−1, the clinically important difference reported in older adults.27,28
For the TUG time, only 1Hz-TT and 25Hz-TT sustained the improvement to 3 months postintervention. The reduced TUG time could be due to faster walking during the 7m walking portion of TUG or overall improvement of all TUG components (ie, sit-to-stand, gait initiation, walking, turning, and termination). Findings of the exploratory analysis showed the reduced time taken to complete the walking portion of TUG was small (Table S2) when compared with that of TUG overall (Table 3). We therefore suggest that our rTMS-primed treadmill training generalized to better ability to perform a sequential mobility task.
The moving treadmill belt provided patients with constant proprioceptive cues, thus allowing for a more rhythmic and normal gait during treadmill training. This resulted in better gait performance in all participants. However, this benefit was more robust in the active rTMS groups and was lost at 1 month postintervention in the sham-TT group. The larger and longer-lasting improvement found in walking and TUG suggests that rTMS boosted activity-dependent plasticity mainly by stabilizing the consolidation process, resulting in long-term retention. rTMS-primed treadmill training could have strengthened synaptic connections within M1, which is involved in processing and storage of new information for motor consolidation,29,30 thus resulting in more stable and prolonged enhancement.
Effect on Motor Automaticity
A long-term improvement in DT-TUG implies better dual-tasking ability and automatic control of movement, which is a significant problem in PD. The striatum plays an important role in acquiring and executing automatic movement.31,32 In addition, efficient neural coding of movement by enhanced network connectivity is essential for the automatic execution of learned motor plans.33 The striatal dopamine depletion in PD would result in difficulty in storing learned automatic skills and acquiring new automatic skills.34 Our rTMS-primed treadmill training protocols could have strengthened the connectivity between the striatum and motor areas, leading to enhanced automaticity during DT-TUG. Notably, only the 25Hz-TT group demonstrated a between-group difference with sham-TT for DT-TUG. A functional magnetic resonance imaging (MRI) study showed that 25Hz rTMS to the bilateral M1 enhanced functional connectivity between the supplementary motor area and prefrontal areas during complex motor tasks.35 Because the supplementary motor area is involved in automatic and complex movement,36 25Hz rTMS may be more effective than sham rTMS in modulating the underactive brain regions and improving motor automaticity in PD.
Effect on PD Motor Symptoms
The benefits of our rTMS-primed treadmill training protocol also translated to alleviation of motor symptoms as measured with MDS-UPDRS-III scores in the long term. This reflects a more widespread improvement in bradykinesia, rigidity, and axial symptoms such as posture, gait, and balance. Our findings concur with a recent study that reported 1Hz and 20Hz rTMS improve motor symptoms in PD.37 We extend previous research by finding that the effects of rTMS-TT on PD motor symptoms last up to 3 months postintervention.8 The interaction between rTMS and treadmill training might induce plastic changes in motor processing within the basal ganglia–thalamocortical circuits38 and/or restore the efficacy of dopamine transmission,39 leading to a long-term improvement in motor symptoms. The improved motor symptoms may also have been augmented by the costimulation of supplementary motor area, which is very close to the stimulation site of M1-leg. Previous studies reported a positive effect of high- or low-frequency rTMS to the supplementary motor area on improving motor symptoms in patients with PD.4 Future research is needed to explore the mechanism underlying such global motor improvement.
Possible Neurophysiological Mechanisms Underlying Behavioral Changes
Impaired motor output or bradykinesia is believed to be the result of insufficient thalamocortical facilitation in PD.38 Functional imaging studies revealed hypoactivity in the supplementary motor area and hyperactivity in bilateral M1 and the premotor cortex.6,40,41 The hyperactive M1 may play a role in motor abnormalities, as shown by a recent finding that there is an association between increased cortical excitability and bradykinesia in PD.42 These investigators proposed that hyperexcitability in M1 may disrupt the encoding of motor parameters, leading to bradykinesia. L-dopa, an antiparkinsonian medication, restores both CSP and SICI, suggesting the involvement of impaired cortical inhibition in the pathophysiology of PD.43,44 The present study showed a positive association between the restoration of cortical inhibition and behavioral improvements only in groups that received real rTMS. These findings suggest that relevant plastic changes at the cortical level have an effect similar to that of dopaminergic medications. The added benefits induced by rTMS for treadmill training may be mediated through the restoration of cortical inhibition, thereby normalizing motor processing. Furthermore, downregulation of cortical hyperexcitability may provide a more propitious brain state for induction of long-term potentiation (LTP), thus promoting neural plasticity critical for long-term functional recovery.
Relative Efficacy of 1Hz and 25Hz rTMS on Priming Treadmill Training
When treadmill training was preceded by 1 or 25Hz rTMS, there were enhanced and prolonged training effects on gait and motor performance compared with sham rTMS. There was no significant difference between the 25Hz-TT and 1Hz-TT groups for all outcomes except TUG at Post1m. The greater improvement from the 25Hz-TT protocol compared to sham-TT is consistent with a previous study in which high-frequency rTMS enhanced the effect of treadmill training,8 implying a possible gating effect of rTMS on subsequent training-induced plasticity.11 Why then would 1Hz rTMS also lead to a better outcome compared to sham rTMS? One possible explanation would be that 1Hz rTMS also increased baseline activity to gate the induction of subsequent LTP induced in treadmill training. Although this postulation disagrees with the conventional view that high-frequency rTMS increases excitability and low-frequency rTMS decreases it, the effects of low-frequency rTMS on cortical excitability in PD has not been confirmed in previous studies.37,45,46 Furthermore, the decreased activation of intracortical inhibition pathways in PD may influence the aftereffect of 1Hz rTMS such that an excitatory effect may outweigh the inhibitory effects.47 Another possible explanation would be that both 25Hz and 1Hz rTMS act to increase cortical inhibitory mechanisms and downregulated the corticomotor hyperexcitability in PD.45,48 However, all the above suggestions only explain the short-term modification in cortical excitability within the homeostatic range.
The important finding of our study is that, for the real rTMS groups, the long-term improvement in gait performance lasted for at least 3 months after the intervention and this improvement is associated with a normalization of cortical excitability. More permanent motor improvement would likely involve a change in the excitability range in the brain. The correlative normalization of cortical excitability demonstrated in our study supports this inference of breaking through the homeostatic excitability barrier by our interventions. Therefore, it is more likely that both 1Hz and 25Hz rTMS exerted gating effects to induce LTP in treadmill training. The cumulative interactions between rTMS and treadmill training eventually broke through the excitability barriers and resulted in nonhomeostatic metaplasticity. Previous research had demonstrated an increase in cortical excitability after a single session of 25Hz rTMS in patients with PD followed by a trend of reduction after the 4-week intervention, suggesting an expansion of plasticity.49
Limitations of the Study
This study has potential limitations. First, our sham rTMS protocol did not generate comparable peripheral sensory stimulation, and the blinding efficacy was not evaluated. Second, more detailed electrophysiological assessments such as stimulus–response curves for CSP and SICI were not performed, considering the patients’ tolerance for prolonged investigation and the short optimal medication therapeutic window. Cortical excitability and intracortical inhibition assessment after the first treadmill training was not feasible for the same reason. Although the lack of this assessment makes it difficult to demonstrate the effect of priming rTMS on metaplasticity after a single training session, it does not preclude the activation of the mechanism involved in the iterative interactions between rTMS and training over consecutive days. In the present study, the correlative normalization of cortical excitability with improved motor performance in the follow-up period implies neuroplastic changes and an alteration in synaptic modification range. Third, costimulation of brain regions other than M1-LEG could not be ruled out, as the double-cone coil was chosen for deeper stimulation, but it has less focality.50 Lastly, the experimental paradigm adopted in this study does not allow us to differentiate the combined effect of rTMS and treadmill training from the priming effect of rTMS. However, this is beyond the scope of this experiment, and future studies should investigate the effect of treatment order. Future studies could employ structural MRI of the brain and a computational model to give more information about the stimulated site, and to simulate the resulting electrical field in the stimulated brain region for establishing the rTMS dose–response relationship. Additional imaging studies or magnetic resonance spectroscopy may assist in confirming the changes in connectivity in the network and change in neural activity of relevant neurotransmitters.
Conclusion
To conclude, the present study extends our understanding of the beneficial effects of rTMS on priming treadmill training in patients with PD and also sheds light on possible neural mechanisms underlying behavioral improvement. Our data demonstrated that both 1Hz and 25Hz rTMS were superior to the sham treatment in consolidating activity-dependent plasticity, leading to long-lasting improvement in fastest walking speed and complex walking tasks in patients with PD. Furthermore, the therapeutic effect of rTMS-primed treadmill training translated to a long-term improvement in motor symptoms and dual-task walking. The motor improvements were associated with a restoration of a cortical inhibition mechanism in the active rTMS groups. Our data suggest that both 1 and 25Hz rTMS promoted treadmill training through gating with a breakthrough of the homeostatic excitability barrier. Rebalancing cortical excitability by rTMS appears critical for plasticity induction in PD.
Supplementary Material
Acknowledgments
This work was supported by the Research Grant Council of the Hong Kong Administrative Region, China (PolyU 15103617). M.H. is supported by the NIH National Institute of Neurological Disorders and Stroke Intramural Program.
Footnotes
View this article online at wileyonlinelibrary.com. DOI: 10.1002/ana.25881
Additional supporting information can be found in the online version of this article.
Potential Conflicts of Interest
Nothing to report.
References
- 1.O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther 2002;82:888–897. [PubMed] [Google Scholar]
- 2.Smulders K, Dale ML, Carlson-Kuhta P, et al. Pharmacological treatment in Parkinson’s disease: effects on gait. Parkinsonism Relat Disord 2016;31:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrholz J, Kugler J, Storch A, et al. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev 2015;2015: CD007830. [DOI] [PubMed] [Google Scholar]
- 4.Chung CL, Mak MK. Effect of repetitive transcranial magnetic stimulation on physical function and motor signs in Parkinson’s disease: a systematic review and meta-analysis. Brain Stimul 2016;9:475–487. [DOI] [PubMed] [Google Scholar]
- 5.Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res Brain Res Rev 2002;38:309–327. [DOI] [PubMed] [Google Scholar]
- 6.Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 2004;14:715–719. [DOI] [PubMed] [Google Scholar]
- 7.Bologna M, Suppa A, Conte A, et al. Are studies of motor cortex plasticity relevant in human patients with Parkinson’s disease? Clin Neurophysiol 2016;127:50–59. [DOI] [PubMed] [Google Scholar]
- 8.Yang YR, Tseng CY, Chiou SY, et al. Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in Parkinson disease: a randomized trial. Neurorehabil Neural Repair 2013;27:79–86. [DOI] [PubMed] [Google Scholar]
- 9.Gilio F, Currà A, Inghilleri M, et al. Repetitive magnetic stimulation of cortical motor areas in Parkinson’s disease: implications for the pathophysiology of cortical function. Mov Disord 2002;17:467–473. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997;48:1398–1403. [DOI] [PubMed] [Google Scholar]
- 11.Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul 2008;1:60–66. [DOI] [PubMed] [Google Scholar]
- 12.Ziemann U, Ilić TV, Jung P. Long-term potentiation (LTP)-like plasticity and learning in human motor cortex—investigations with transcranial magnetic stimulation (TMS). Suppl Clin Neurophysiol 2006; 59:19–25. [DOI] [PubMed] [Google Scholar]
- 13.Hamada M, Terao Y, Hanajima R, et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol 2008;586:3927–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2008;119:504–532. [DOI] [PubMed] [Google Scholar]
- 16.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120: 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancini M, King L, Salarian A, et al. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci 2011;1:007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 19.Madhavan S, Rogers LM, Stinear JW. A paradox: after stroke, the non-lesioned lower limb motor cortex may be maladaptive. Eur J Neurosci 2010;32:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 2004;115:1076–1082. [DOI] [PubMed] [Google Scholar]
- 21.Poston B, Kukke SN, Paine RW, et al. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur J Neurosci 2012;36:2964–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2012;123:858–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord 2009;15:S53–S58. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005;128:2250–2259. [DOI] [PubMed] [Google Scholar]
- 26.Chui K, Hood E, Klima D. Meaningful change in walking speed. Top Geriatr Rehabil 2012;28:97–103. [Google Scholar]
- 27.Hardy SE, Perera S, Roumani YF, et al. Improvements in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 2007;55:1727–1734. [DOI] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muellbacher W, Ziemann U, Boroojerdi B, et al. Role of the human motor cortex in rapid motor learning. Exp Brain Res 2001;136: 431–438. [DOI] [PubMed] [Google Scholar]
- 30.Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 2009;106: 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehéricy S, Benali H, Van de Moortele PF, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A 2005;102:12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieuwhof F, Bloem BR, Reelick MF, et al. Impaired dual tasking in Parkinson’s disease is associated with reduced focusing of corticostriatal activity. Brain 2017;140:1384–1398. [DOI] [PubMed] [Google Scholar]
- 33.Wu T, Chan P, Hallett M. Modifications of the interactions in the motor network when a movement becomes automatic. J Physiol 2008;586:4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis 2015;82:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-García N, Armony JL, Soto J, et al. Effects of rTMS on Parkinson’s disease: a longitudinal fMRI study. J Neurol 2011;258: 1268–1280. [DOI] [PubMed] [Google Scholar]
- 36.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996;6:342–353. [DOI] [PubMed] [Google Scholar]
- 37.Khedr EM, Al-Fawal B, Abdel Wraith A, et al. The effect of 20 Hz versus 1 Hz repetitive transcranial magnetic stimulation on motor dysfunction in Parkinson’s disease: which is more beneficial? J Parkinson’s Dis 2019;9:379–387. [DOI] [PubMed] [Google Scholar]
- 38.DeLong MR, Wichmann T. Basal ganglia circuits as targets for neuromodulation in Parkinson disease. JAMA Neurol 2015;72: 1354–1360. [DOI] [PubMed] [Google Scholar]
- 39.Pirker W Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord 2003;18: S43–S51. [DOI] [PubMed] [Google Scholar]
- 40.Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 2000;123:394–403. [DOI] [PubMed] [Google Scholar]
- 41.Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol 2005;116: 244–253. [DOI] [PubMed] [Google Scholar]
- 42.Bologna M, Guerra A, Paparella G, et al. Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain 2018;141: 2432–2444. [DOI] [PubMed] [Google Scholar]
- 43.Priori A, Berardelli A, Inghilleri M, et al. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain 1994; 117:317–323. [DOI] [PubMed] [Google Scholar]
- 44.Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol 1995;37:181–188. [DOI] [PubMed] [Google Scholar]
- 45.Lefaucheur JP, Drouot X, Von Raison F, et al. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol 2004;115:2530–2541. [DOI] [PubMed] [Google Scholar]
- 46.Sommer M, Kamm T, Tergau F, et al. Repetitive paired-pulse transcranial magnetic stimulation affects corticospinal excitability and finger tapping in Parkinson’s disease. Clin Neurophysiol 2002;113: 944–950. [DOI] [PubMed] [Google Scholar]
- 47.Siebner HR, Auer C, Conrad B. Abnormal increase in the corticomotor output to the affected hand during repetitive transcranial magnetic stimulation of the primary motor cortex in patients with writer’s cramp. Neurosci Lett 1999;262:133–136. [DOI] [PubMed] [Google Scholar]
- 48.Fierro B, Brighina F, D’Amelio M, et al. Motor intracortical inhibition in PD: L-DOPA modulation of high-frequency rTMS effects. Exp Brain Res 2008;184:521–528. [DOI] [PubMed] [Google Scholar]
- 49.Lomarev MP, Kanchana S, Bara-Jimenez W, et al. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord 2006;21:325–331. [DOI] [PubMed] [Google Scholar]
- 50.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol 2002;19: 361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


